
ЁОЬтФПЁПФГбаОПадбЇЯАаЁзщЩшМЦСЫШчЯТзАжУжЦШЁКЭбщжЄSO2ЕФаджЪ
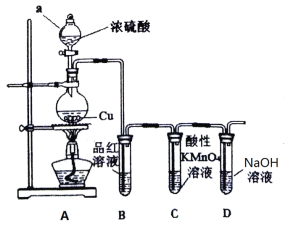
ЧыЛиД№ЃК
ЃЈ1ЃЉЭМжавЧЦїaЕФУћГЦЮЊ_____________ЁЃ
ЃЈ2ЃЉAжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЪЧ_____________________________________ЁЃ
ЃЈ3ЃЉШєCжаШмвКЭЪЩЋЃЌЬхЯжSO2Опга_________________адЁЃ
ЃЈ4ЃЉЙ§СПNaOHШмвКгыSO2ЗДгІЕФРызгЗНГЬЪНЪЧ__________________________________ЁЃ
ЃЈ5ЃЉЯђСНжЇзАгаЦЗКьШмвКЕФЪдЙмжаЃЌЗжБ№ЭЈШыCl2КЭSO2ЃЌЗЂЯжСНжЇЪдЙмжаЦЗКьШмвКОљЭЪЩЋЃЌЧыФуЩшМЦЪЕбщжЄУїФФжЇЪдЙмжаЭЈШыЕФЪЧSO2ЃК_________________ЁЃ
ЃЈ6ЃЉИУаЁзщМЬајЩшМЦЪЕбщЃЌЯрЭЌЬѕМўЯТНЋCl2КЭSO2СНжжЦјЬхАДЬхЛ§1ЃК1ЛьКЯЃЌдйЭЈШыЦЗКьШмвКжаЃЌЙлВьШмвКМИКѕВЛЭЪЩЋЁЃВщдФзЪСЯжЊЃКСНжжЦјЬхАДЬхЛ§1ЃК1ЛьКЯЃЌдйгыЫЎЗДгІПЩЩњГЩСНжжГЃМћЕФЫсЃЌвђЖјЪЇШЅЦЏАззїгУЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЪЧ________________________ЁЃ
ЁОД№АИЁПЗжвКТЉЖЗ Cu + 2H2SO4 (ХЈ) ![]() CuSO4 + SO2Ёќ+2H2O ЛЙд SO2+2OH= SO32 + H2O НЋСНжЇЪдЙмжаЕФвКЬхМгШШЃЌЛжИДКьЩЋЕФЭЈШыЕФЮЊSO2 Cl2+SO2+2H2O=H2SO4+2HCl
CuSO4 + SO2Ёќ+2H2O ЛЙд SO2+2OH= SO32 + H2O НЋСНжЇЪдЙмжаЕФвКЬхМгШШЃЌЛжИДКьЩЋЕФЭЈШыЕФЮЊSO2 Cl2+SO2+2H2O=H2SO4+2HCl
ЁОНтЮіЁП
AжаХЈСђЫсгыЭдкМгШШЬѕМўЯТЗДгІЩњГЩЖўбѕЛЏСђКЭЫЎЁЂСђЫсЭЃЌЖўбѕЛЏСђОпгаЦЏАзадЃЌФмЙЛЪЙBжаЦЗКьШмвКЭЪЩЋЃЌЖўбѕЛЏСђОпгаЛЙдадЃЌФмЙЛгыCжаИпУЬЫсМиШмвКЭЪЩЋЃЌЖўбѕЛЏСђгаЖОЃЌЖргрЕФЖўбѕЛЏСђПЩвдгУDжаЧтбѕЛЏФЦЮќЪеЃЌвдДЫНтД№ИУЬтЁЃ
ЃЈ1ЃЉгЩзАжУЭМПЩжЊвЧЦїaЮЊЗжвКТЉЖЗЃЌЙЪД№АИЮЊЃКЗжвКТЉЖЗЃЛ
ЃЈ2ЃЉХЈСђЫсОпгаЧПбѕЛЏадЃЌМгШШЬѕМўЯТПЩгыЭЗЂЩњбѕЛЏЛЙдЗДгІЃЌЗНГЬЪНЮЊ![]() ЃЌ
ЃЌ
ЃЈ3ЃЉИпУЬЫсМиОпгаЧПбѕЛЏадЃЌCжаИпУЬЫсМиЭЪЩЋЃЌЫЕУїЖўбѕЛЏСђОпгаЛЙдадЃЌЙЪД№АИЮЊЃКЛЙдЃЛ
ЃЈ4ЃЉЙ§СПNaOHШмвКгыSO2ЗДгІЩњГЩбЧСђЫсФЦЃЌРызгЗНГЬЪНЪЧSO2+2OH-=SO32-+H2OЃЛ
ЃЈ5ЃЉЖўбѕЛЏСђЕФЦЏАзаЇЙћОпгаВЛЮШЖЈадЃЌМгШШПЩЛжИДЕНдРДЕФбеЩЋЃЌдђМјБ№СНжжЦјЬхЃЌПЩНЋСНжЇЪдЙмжаЕФвКЬхМгШШЃЌЛжИДКьЩЋЕФЭЈШыЕФЮЊSO2ЃЌЙЪД№АИЮЊЃКНЋСНжЇЪдЙмжаЕФвКЬхМгШШЃЌЛжИДКьЩЋЕФЭЈШыЕФЮЊSO2ЃЛ
ЃЈ6ЃЉТШЦјгыЖўбѕЛЏСђЗЂЩњбѕЛЏЛЙдЗДгІЩњГЩСђЫсКЭбЮЫсЃЌЗДгІЕФЗНГЬЪНЮЊCl2+SO2+2H2O=H2SO4+2HClЁЃ


 ГЌФмбЇЕфгІгУЬтЬтПЈЯЕСаД№АИ
ГЌФмбЇЕфгІгУЬтЬтПЈЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПввЫсввѕЅЙуЗКгУгквЉЮяЁЂШМСЯЁЂЯуСЯЕШЙЄвЕЃЌдкжабЇЛЏбЇЪЕбщЪвРяГЃгУШчЭМзАжУРДжЦБИввЫсввѕЅЁЃ![]() ВПЗжМаГжвЧЦївбТдШЅ
ВПЗжМаГжвЧЦївбТдШЅ![]()
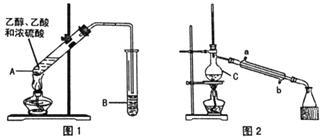
вбжЊЃК
УмЖШ(g/cm3) | ШлЕу(Ёц) | ЗаЕу(Ёц) | ШмНтЖШ | |
ввДМ | 0.79 | -114.5 | 78.4 | гыЫЎЛЅШм |
ввЫс | 1.05 | 16.6 | 118.1 | взШмгкЫЎЁЂввДМ |
ввЫсввѕЅ | 0.90 | -83.6 | 77.2 | ЮЂШмгкЫЎЃЌФмШмгкввДМ |
ЂёжЦБИДжЦЗ(ЭМ1)
дкAжаМгШыЩйСПЫщДЩЦЌЃЌНЋШ§жждСЯвРДЮМгШыAжаЃЌгУОЦОЋЕЦЛКТ§МгШШЃЌвЛЖЮЪБМфКѓдкBжаЕУЕНввЫсввѕЅДжЦЗЁЃ
ЃЈ1ЃЉХЈСђЫсЁЂввДМЁЂввЫсЕФМгШыЫГађЪЧ___ЃЌAжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЪЧ___ЁЃ
ЃЈ2ЃЉAжаЫщДЩЦЌЕФзїгУЪЧ___ЃЌГЄЕМЙмГ§СЫЕМЦјЭтЃЌЛЙОпгаЕФзїгУЪЧ___ЁЃ
ЃЈ3ЃЉBжаЪЂзАЕФвКЬхЪЧ___ЃЌЪеМЏЕНЕФввЫсввѕЅдк___Ву(ЬюЁАЩЯЁБЛђЁАЯТЁБ)ЁЃ
Ђђ.жЦБИОЋЦЗ(ЭМ2)
НЋBжаЕФвКЬхЗжвКЃЌЖдввЫсввѕЅДжЦЗНјаавЛЯЕСаГ§дгВйзїКѓзЊвЦЕНCжаЃЌРћгУЭМ2зАжУНјвЛВНВйзїМДЕУЕНввЫсввѕЅОЋЦЗЁЃ
ЃЈ4ЃЉCЕФУћГЦЪЧ___ЁЃ
ЃЈ5ЃЉЪЕбщЙ§ГЬжаЃЌРфШДЫЎДг___ПкНјШы(ЬюзжФИ)ЃЛЪеМЏВњЦЗЪБЃЌПижЦЕФЮТЖШгІдк___зѓгвЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖўбѕЛЏЕЊПЩгЩNOКЭO2ЩњГЩЃЌвбжЊдк2LУмБеШнЦїФкЃЌ800ЁцЪБЗДгІЃК2NO(g)ЃЋO2(g)![]() 2NO2(g) ІЄHЃЌn(NO)ЁЂn(O2)ЫцЪБМфЕФБфЛЏШчБэЃК
2NO2(g) ІЄHЃЌn(NO)ЁЂn(O2)ЫцЪБМфЕФБфЛЏШчБэЃК
ЪБМф/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.200 | 0.100 | 0.080 | 0.050 | 0.050 | 0.050 |
n(O2)/mol | 0.100 | 0.050 | 0.040 | 0.025 | 0.025 | 0.025 |
ЃЈ1ЃЉвбжЊЃКK800Ёц>K1000ЁцЃЌдђИУЗДгІЕФІЄH___0(ЬюЁАДѓгкЁБЛђЁАаЁгкЁБ)ЃЌгУO2БэЪО0ЁЋ2 sФкИУЗДгІЕФЦНОљЫйТЪЮЊ___ЁЃ
ЃЈ2ЃЉФмЫЕУїИУЗДгІвбДяЕНЦНКтзДЬЌЕФЪЧ___ЁЃ
aЃЎШнЦїФкЦјЬхбеЩЋБЃГжВЛБф bЃЎ2vФц(NO)ЃНvе§(O2)
cЃЎШнЦїФкбЙЧПБЃГжВЛБф dЃЎШнЦїФкЦјЬхУмЖШБЃГжВЛБф
ЃЈ3ЃЉЮЊЪЙИУЗДгІЕФЫйТЪдіДѓЃЌЬсИпNOЕФзЊЛЏТЪЃЌЧвЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌгІВЩШЁЕФДыЪЉга_____ЁЃ
ЃЈ4ЃЉдкЬтЪіЬѕМўЯТЃЌМЦЫуЭЈШы2molNOКЭ1molO2ЕФЦНКтГЃЪ§KЃН___ЁЃ
ЃЈ5ЃЉдкЬтЪіЬѕМўЯТЃЌШєПЊЪМЭЈШыЕФЪЧ0.2molNO2ЦјЬхЃЌДяЕНЛЏбЇЦНКтЪБЃЌNO2ЕФзЊЛЏТЪЮЊ__ЁЃ
ЃЈ6ЃЉУКШМЩеВњЩњЕФбЬЦјКЌЕЊЕФбѕЛЏЮяЃЌгУCH4ДпЛЏЛЙдNOxПЩвдЯћГ§ЕЊбѕЛЏЮяЕФЮлШОЁЃ
ЂйCH4(g)ЃЋ4NO(g)![]() 2N2(g)ЃЋCO2(g)ЃЋ2H2O ІЄH<0
2N2(g)ЃЋCO2(g)ЃЋ2H2O ІЄH<0
ЂкCH4(g)ЃЋ2NO2(g)![]() N2(g)ЃЋCO2(g)ЃЋ2H2O(g) ІЄH<0
N2(g)ЃЋCO2(g)ЃЋ2H2O(g) ІЄH<0
ЖдгкЗДгІЂкЃЌгћЬсИпNO2ЕФзЊЛЏТЪЃЌПЩВЩШЁЕФДыЪЉга____ЁЃ
aЃЎдіМгдДпЛЏМСЕФБэУцЛ§ bЃЎНЕЕЭЮТЖШ cЃЎМѕаЁЭЖСЯБШ[n(NO2)/n(CH4)] dЃЎдіДѓбЙЧП
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖўбѕЛЏЙшОЇЬхЪЧСЂЬхЕФЭјзДНсЙЙЃЌЦфОЇЬхНсЙЙФЃаЭШчЭМЁЃЧыШЯецЙлВьИУФЃаЭКѓЛиД№ЯТСаЮЪЬтЃК
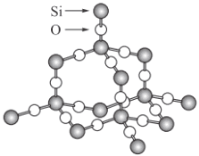
(1)ЖўбѕЛЏЙшОЇЬхжазюаЁЛЗЩЯга______ИідзгЃЌОЇЬхНсЙЙжаДцдквд__________дзгЮЊжааФЁЂ________дзгЮЊЖЅЕуЕФе§ЫФУцЬхНсЙЙЁЃ
(2)ОЇЬхжаДцдкЕФзїгУСІга________ЁЃ
AЃЎЙВМлМќЁЁBЃЎРызгМќЁЁCЃЎХфЮЛМќЁЁDЃЎЗЖЕТЛЊСІЁЁEЃЎЧтМќ
(3)УРЙњLawreceLiremoreЙњМвЪЕбщЪв(LLNL)ЕФV.Lota.C.S.YooКЭCynnГЩЙІЕидкИпбЙЯТНЋCO2зЊЛЏЮЊОпгаРрЫЦSiO2НсЙЙЕФдзгОЇЬхЃЌЯТСаЙигкCO2ЕФдзгОЇЬхЫЕЗЈе§ШЗЕФЪЧ________ЁЃ
AЃЎCO2ЕФдзгОЇЬхКЭЗжзгОЇЬхЛЅЮЊЭЌЫивьаЮЬх
BЃЎдквЛЖЈЬѕМўЯТЃЌCO2дзгОЇЬхзЊЛЏЮЊЗжзгОЇЬхЪЧЮяРэБфЛЏ
CЃЎCO2ЕФдзгОЇЬхКЭCO2ЕФЗжзгОЇЬхОпгаЯрЭЌЕФЮяРэаджЪ
DЃЎдкCO2ЕФдзгОЇЬхжаЃЌУПИіCдзгНсКЯ4ИіOдзгЃЌУПИіOдзгНсКЯСНИіCдзг
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдзгНсЙЙгыдЊЫижмЦкБэДцдкзХФкдкСЊЯЕЁЃИљОнЫљбЇЮяжЪНсЙЙжЊЪЖЃЌЧыФуЛиД№ЯТСаЮЪЬтЃК
(1)ЧыАДвЊЧѓШЮвтаДвЛИіЯргІЮяжЪЃК(ЬюЛЏбЇЪН)КЌгаЗЧМЋадМќЕФРызгЛЏКЯЮя________ЃЌМШКЌгаЗЧМЋадМќгжКЌМЋадМќЕФЗЧМЋадЗжзг________ЃЌМШКЌгаЗЧМЋадМќгжКЌМЋадМќЕФМЋадЗжзг________ЃЌШЋВПгЩЗЧН№ЪєдЊЫизщГЩЕФРызгЛЏКЯЮя________ЃЌгЩН№ЪєдЊЫиКЭЗЧН№ЪєдЊЫизщГЩЕФЙВМлЛЏКЯЮя________ЁЃ
(2)ЫеЕЄКьбеЩЋЯЪбоЁЂМлИёЕЭСЎЃЌГЃБЛвЛаЉЦѓвЕЗЧЗЈзїЮЊЪГЦЗКЭЛЏзБЦЗЕШЕФШОЩЋМСЃЌбЯжиЮЃКІШЫУЧНЁПЕЁЃЫеЕЄКьГЃМћгаЂёЁЂЂђЁЂЂѓЁЂЂє4жжРраЭЃЌЫеЕЄКьЂёЕФЗжзгНсЙЙШчЭМЫљЪОЁЃ


ЫеЕЄКьЂёдкЫЎжаЕФШмНтЖШКмаЁЃЌЮЂШмгкввДМЃЌгаШЫАбєЧЛљШЁДњдкЖдЮЛаЮГЩЭМЫљЪОЕФНсЙЙЃЌдђЦфдкЫЎжаЕФШмНтЖШЛс________(ЬюЁАдіДѓЁБЛђЁАМѕаЁЁБ)ЃЌдвђЪЧ_____________________________________ЁЃ
(3)вбжЊTi3ЃЋПЩаЮГЩХфЮЛЪ§ЮЊ6ЃЌбеЩЋВЛЭЌЕФСНжжХфКЯЮяОЇЬхЃЌвЛжжЮЊзЯЩЋЃЌСэвЛжжЮЊТЬЩЋЁЃСНжжОЇЬхЕФзщГЩНдЮЊTiCl3ЁЄ6H2OЁЃЮЊВтЖЈетСНжжОЇЬхЕФЛЏбЇЪНЃЌЩшМЦСЫШчЯТЪЕбщЃК
aЃЎЗжБ№ШЁЕШжЪСПЕФСНжжХфКЯЮяОЇЬхЕФбљЦЗХфГЩД§ВтШмвКЃЛ
bЃЎЗжБ№ЭљД§ВтШмвКжаЕЮШыAgNO3ШмвКЃЌОљВњЩњАзЩЋГСЕэЃЛ
cЃЎГСЕэЭъШЋКѓЗжБ№Й§ТЫЕУСНЗнГСЕэЃЌОЯДЕгИЩдяКѓГЦСПЃЌЗЂЯждТЬЩЋОЇЬхЕФЫЎШмвКЕУЕНЕФАзЩЋГСЕэжЪСПЮЊдзЯЩЋОЇЬхЕФЫЎШмвКЕУЕНЕФГСЕэжЪСПЕФ2/3ЁЃдђТЬЩЋОЇЬхХфКЯЮяЕФЛЏбЇЪНЮЊ________________ЃЌгЩClЃЫљаЮГЩЕФЛЏбЇМќРраЭЪЧ________ЁЃ
(4)ЯТЭМжаAЃЌBЃЌCЃЌDЫФЬѕЧњЯпЗжБ№БэЪОЕкЂєAЁЂЂѕAЁЂЂіAЁЂЂїAзхдЊЫиЕФЧтЛЏЮяЕФЗаЕуЃЌЦфжаБэЪОЂїAзхдЊЫиЧтЛЏЮяЗаЕуЕФЧњЯпЪЧ________ЃЛБэЪОЂєAзхдЊЫиЧтЛЏЮяЗаЕуЕФЧњЯпЪЧ________ЃЛЭЌвЛзхжаЕк3ЁЂ4ЁЂ5жмЦкдЊЫиЕФЧтЛЏЮяЗаЕувРДЮЩ§ИпЃЌЦфдвђЪЧ_____________________________ЃЛAЃЌBЃЌCЧњЯпжаЕкЖўжмЦкдЊЫиЕФЧтЛЏЮяЕФЗаЕуЯджјИпгкЕкШ§жмЦкдЊЫиЕФЧтЛЏЮяЕФЗаЕуЃЌЦфдвђЪЧ_______________________ЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПН№ОЇЬхЕФзюаЁжиИДЕЅдЊ(вВГЦОЇАћ)ЪЧУцаФСЂЗНЬхЃЌМДдкСЂЗНЬхЕФ8ИіЖЅЕуИїгавЛИіН№дзгЃЌИїИіУцЕФжааФгавЛИіН№дзгЃЌУПИіН№дзгБЛЯрСкЕФОЇАћЙВгУЁЃН№дзгЕФжБОЖЮЊdcmЃЌгУNAБэЪОАЂЗќМгЕТТоГЃЪ§ЃЌMБэЪОН№ЕФФІЖћжЪСП(ЕЅЮЛЃКgЁЄmolЃ1)ЁЃ
(1)Н№ОЇЬхУПИіОЇАћжаКЌга________ИіН№дзгЁЃ
(2)гћМЦЫувЛИіОЇАћЕФЬхЛ§ЃЌГ§МйЖЈН№дзгЪЧИеадаЁЧђЭтЃЌЛЙгІМйЖЈ_________ЁЃ
(3)вЛИіОЇАћЕФЬхЛ§ЮЊ________cm3ЁЃ
(4)Н№ОЇЬхЕФУмЖШЮЊ________gЁЄcmЃ3ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПГЃЮТЯТЃЌЗжБ№ЕїНкХЈЖШОљЮЊ0.1 molЁЄLЃ1 HAШмвКЁЂHBЕФШмвКЕФpHЃЌЫљЕУШмвКжаЫсЗжзгЕФАйЗжКЌСПІи%(ШчHAЕФАйЗжКЌСПЮЊ![]() ЁС100ЃЅ)гыpHЕФБфЛЏЙиЯЕШчЭМЫљЪОЁЃвбжЊЕїНкpHЪБВЛгАЯьШмвКзмЬхЛ§ЁЃЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
ЁС100ЃЅ)гыpHЕФБфЛЏЙиЯЕШчЭМЫљЪОЁЃвбжЊЕїНкpHЪБВЛгАЯьШмвКзмЬхЛ§ЁЃЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
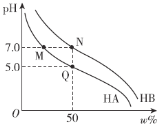
A.ГЃЮТЯТЃЌKa(HA)ЃН1.0ЁС10Ѓ5B.MЁЂNСНЕуЖдгІРызгХЈЖШЃКc(AЃ)ЃНc(BЃ)
C.НЋMЁЂNСНЕуШмвКЕШЬхЛ§ЛьКЯЃЌШмвКГЪжаадD.ЫЎЕФЕчРыГЬЖШЃКMЃНN>Q
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПДпЛЏЛЙдCO2ЪЧНтОіЮТЪваЇгІМАФмдДЮЪЬтЕФживЊЪжЖЮжЎвЛЁЃбаОПБэУїЃЌдкCu/ZnOДпЛЏМСДцдкЯТЃЌCO2КЭH2ПЩЗЂЩњСНИіЦНааЗДгІЃЌЗжБ№ЩњГЩCH3OHКЭCOЁЃЗДгІЕФШШЛЏбЇЗНГЬЪНШчЯТЃК
CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ІЄH1=-53.7kJЁЄmol-1 I
CH3OH(g)+H2O(g) ІЄH1=-53.7kJЁЄmol-1 I
CO2(g)+H2(g)![]() CO(g)+H2O(g) ІЄH2 II
CO(g)+H2O(g) ІЄH2 II
ФГЪЕбщЪвПижЦCO2КЭH2ГѕЪМЭЖСЯБШЮЊ1ЃК2.2ЃЌдкЯрЭЌбЙЧПЯТЃЌОЙ§ЯрЭЌЗДгІЪБМфВтЕУШчЯТЪЕбщЪ§ОнЃК
T(K) | ДпЛЏМС | CO2зЊЛЏТЪЃЈ%ЃЉ | МзДМбЁдёадЃЈ%ЃЉ |
543 | Cat.1 | 12.3 | 42.3 |
543 | Cat.2 | 10.9 | 72.7 |
553 | Cat.1 | 15.3 | 39.1 |
553 | Cat.2 | 12.0 | 71.6 |
ЃЈБИзЂЃЉCat.1ЃКCu/ZnOФЩУзАєЃЛCat.2ЃКCu/ZnOФЩУзЦЌЃЛМзДМбЁдёадЃКзЊЛЏЕФCO2жаЩњГЩМзДМЕФАйЗжБШ
вбжЊЃКЂйCOКЭH2ЕФБъзМШМЩеШШЗжБ№ЮЊ-283.0kJЁЄmol-1КЭ-285.8kJЁЄmol-1
ЂкH2O(l)=H2O(g) ІЄH3=44.0kJЁЄmol-1
ЧыЛиД№ЃЈВЛПМТЧЮТЖШЖдІЄHЕФгАЯьЃЉЃК
(1)ЗДгІIЕФЦНКтГЃЪ§БэДяЪНK=___ЃЛ
(2)гаРћгкЬсИпCO2зЊЛЏЮЊCH3OHЦНКтзЊЛЏТЪЕФДыЪЉга___ЁЃ
AЃЎЪЙгУДпЛЏМСCat.1
BЃЎЪЙгУДпЛЏМСCat.2
CЃЎНЕЕЭЗДгІЮТЖШ
DЃЎЭЖСЯБШВЛБфЃЌдіМгЗДгІЮяЕФХЈЖШ
EЃЎдіДѓCO2КЭH2ЕФГѕЪМЭЖСЯБШ
(3)БэжаЪЕбщЪ§ОнБэУїЃЌдкЯрЭЌЮТЖШЯТВЛЭЌЕФДпЛЏМСЖдCO2зЊЛЏГЩCH3OHЕФбЁдёадгаЯджјЕФгАЯьЃЌЦфдвђЪЧ___ЁЃ
(4)дкЭМжаЗжБ№ЛГіЗДгІIдкЮоДпЛЏМСЁЂгаCat.1КЭгаCat.2Ш§жжЧщПіЯТЁАЗДгІЙ§ГЬЁЋФмСПЁБЪОвтЭМ___ЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдквЛЖЈЬѕМўЯТЃЌЗДгІ2AЃЈgЃЉ+2BЃЈgЃЉ![]() 3CЃЈgЃЉ+DЃЈgЃЉдкКуШнШнЦїжаНјааЃЌДяЕНЛЏбЇЦНКтЕФБъжОЪЧЃЈ ЃЉ
3CЃЈgЃЉ+DЃЈgЃЉдкКуШнШнЦїжаНјааЃЌДяЕНЛЏбЇЦНКтЕФБъжОЪЧЃЈ ЃЉ
A. ЕЅЮЛЪБМфФкЩњГЩ2n molBЃЌЭЌЪБЯћКФ3n molCB. ШнЦїФкбЙЧПВЛЫцЪБМфБфЛЏ
C. ЛьКЯЦјЬхЕФУмЖШВЛЫцЪБМфБфЛЏD. BЮяжЪЕФАйЗжКЌСПВЛБф
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com