
X��Y��Z��M��G����Ԫ�ط�������������,��ԭ��������������X��Zͬ����,���γ����ӻ�����ZX ; Y��Mͬ����,���γ�MY2��MY3���ַ��ӡ�
��1��Y��Ԫ�����ڱ��е�λ��Ϊ���������������� ��
��2������Ԫ�ص�����������Ӧ��ˮ����������ǿ������������ (д��ѧʽ),�ǽ�����̬�⻯�ﻹԭ����ǿ������������ (д��ѧʽ)��
��3��X��Y��Z��M��������ε���Һ��Ӧ�ɲ���MY2���壬д���䷴Ӧ���ӷ���ʽ������������������
��4��M����������G�ĵ��ʵ�ˮ��Һ����Ư���ԣ���ͬ�����£���ͬ�����M����������Y�ĵ��ʻ��ͨ��Ʒ����Һ��Ʒ����Һ������������ɫ����ɫ����ԭ���û�ѧ����ʽ��ʾ������������������
��1���ڶ�����VIA�� ��2��HClO4 H2S ��3��HSO3-+H+ = SO2��+ H2O
��4������ɫ �� Cl2+SO2 +2H2O =H2SO4 +2HCl
���������������1���������֪X��H��Y��O��Z��Na��M��S��G��Cl����Y��Ԫ�����ڱ��е�λ����λ�ڵڶ�����VIA�壬��2�����ڷǽ�������ǿ��Ԫ����Cl������������������Ӧ��ˮ����������ǿ����HClO4���ǽ�����̬�⻯�ﻹԭ����ǿ����H2S����3��MY2ΪSO2����������Ԫ����ɵ���������ΪNaHSO4��NaHSO3��NaHSO4��ˮ��Һ�пɵ����H+��һԪǿ������ã���Ӧ�����ӷ���ʽΪ��HSO3-+H+ = SO2��+ H2O����4�� )M����Ư���Ե�������ΪSO2��G�ĵ���ΪCl2����ΪSO2�л�ԭ�� ��Cl2�������ԡ�������1:1�����ʵ����ıȻ�ϣ���ˮ�з���ǡ��������ԭ��ӦCl2+ SO2+ 2H2O=2HCl+H2SO4������û��Ư���Ե�HCl��H2SO4�����ʧȥƯ��������
���㣺����Ԫ�ص��ƶϼ�SO2��Cl2��Ư���ԡ�H2S��ԭ�Ե����ʵ�֪ʶ��


 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���л�ѧʵ����ʵ������۶���ȷ���ǣ� ��
| ѡ�� | ʵ����ʵ | ���� |
| A | ��SO2ͨ�뺬HClO����Һ������H2SO4 | HClO�����Ա�H2SO4ǿ |
| B | �����ھƾ��ƻ����ϼ����ۻ��������� | ���������������۵������ |
| C | SiO2���Ժ�NaOH��Һ��HF��Һ��Ӧ | SiO2�������������� |
| D | ��SO2ͨ����ˮ�У���ˮ��ɫ | SO2����Ư���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���������̿���Ҫ�ɷ�ΪMnO2��ͬʱ�������������ȵĻ�����Ʊ������̵ij����������£�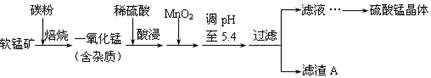
���ֽ���������������������ʽ��ȫ����ʱ��Һ��pH���±���
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 10.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij���Ȼ�����A�����ֶ�����Ԫ����ɣ������¸�����Ϊ��̬����ø�����Կ���������ܶ�Ϊ3.0��A����ˮ�ɵ�ֻ����һ����B����������Һ��B��Һ�ڷ��ù����������Ի���ǿ�������£�����A��NH3��Ӧ�������Ӿ���C�����嵥��D�ͳ���Һ��E��DΪ�����к����������ʡ�����A����ijһ���嵥���볱ʪ��Na2CO3��Ӧ�Ƶã�ͬʱ�����������Ρ���ش��������⣺
��1������A�Ļ�ѧʽΪ �����嵥��D��ӦԪ�������ڱ��е�λ��Ϊ ��
��2���û�ѧ����ʽ��ʾB��Һ������ǿ��ԭ�� ��
��3������A��NH3��Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ��������A���� �ԡ�
��4����д����ȡ����A�Ļ�ѧ����ʽΪ ��
��5�����ʵ��̽�����Ӿ���C�ijɷ�Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��11�֣���C��Cu��FeCO3��ͭ��[��Ҫ�ɷ�ΪCu2(OH)2CO3]��ɵĹ������������������ʾ��ʵ����̣�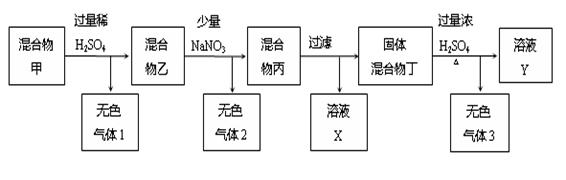
�ش��������⣺
��1����ɫ����1�ijɷ��ǣ� ��
��2����������м���NaNO3��д��һ�����ܲ�����ɫ����2�ķ�Ӧ�����ӷ���ʽ��
��3����ҺX�к��еĽ����������� ��
��4����ɫ����3�ɷ��� ����֪�������ﶡ�ĵ�����Ϊ5.6g���ڱ�״������ɫ����3�����Ϊ14.56L������ɫ����3���ɷֵ����ʵ����� ����д������ļ�����̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ԫ��A��B��M��ɵ��������ʷ�����Ӧ:��+��=��+��,���м���A��M���,����B��M���,��ֻ����M��
��1������Ϊ����ɫ���壬�Һͱ���Ϊ�����µ���ɫ��ζ���塣���ҵĵ���ʽΪ ;���ɱ�״����5.6L��ת�Ƶĵ�����Ϊ ;�����¶���ҺpH 7,�����ӷ���ʽ���� ��
��2������Ϊ��ʹƷ����ɫ����ɫ���壬��Ϊ������ɫ������������ס�����ԭ�Ӹ����Ⱦ�Ϊ1:2(M����+1��),ԭ������B����A�����A�����ڱ���λ��Ϊ �ڶ���ˮ��Ӧ�Ļ�ѧ����ʽΪ ��Ӧ����Һ�еμ�������ɫʯ����Һ������Ϊ
����ȷ��д�������ɱ��Ļ�ѧ����ʽ
����MCl2����Һ��ͨ�붡,�ɹ۲쵽��ɫ��MCl����,д���÷�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ֿ���������A��B��C��D��E���������������������ӻ�����ͬ���ֱ�������������Na+��Al3+��Mg2+��Ba2+��Fe3+������������Cl?��OH?��NO3?��CO ��X�е�һ�֡�
��X�е�һ�֡�
��1��ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������
���ѧʽ����
��2��Ϊ��ȷ��X���ֽ���1���е��������ʼ�ΪA��B����C��B����Һ���ʱ���������ɫ��������ɫ��ζ���壻��C��A����Һ���ʱ�����ػ�ɫ��������ó����е���ϡHNO3�����������ܽ⣬������а�ɫ���������ܽ⡣��
��XΪ ��
A��SO B��SO
B��SO C��CH3COO D��SiO
C��CH3COO D��SiO
��A�еĻ�ѧ������Ϊ ��
�۽�0.02 mol��A��0.01mol��Cͬʱ�ܽ�������������ˮ�У���ַ�Ӧ���������ó���������Ϊ ����ȷ��0.1g)��
�����������Ѿ�ȷ�������ʣ����Լ����D��E�е������ӡ������ʵ��������衢������ ��
��3����CuͶ�뵽װ��D��Һ���Թ��У�Cu���ܽ⣻�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֣���÷�Ӧ�����ӷ���ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��Q��W��R���ֶ�����Ԫ��ԭ���������������������X��Z��Q����Ԫ����ɣ�������0.1mol/L����Һ��pH��13����ҵ�ϳ��õ�ⱥ��QR��Һ���ɼף�����������X��R����Ԫ����ɡ���ش��������⣺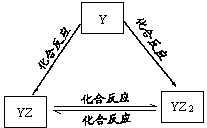
��1��Q��ԭ�ӽṹʾ��ͼΪ ��
��2��YԪ�صĵ����ܷ�����ͼ��ʾ��ת������YԪ��Ϊ ����Ԫ�ط��ű�ʾ�����ڼ���Һ��ͨ������YZ2���壬������Һ�ʼ��ԣ�ԭ���� �������ӷ���ʽ�ͱ�Ҫ������˵������
��3��W�ĵ��ʼ��������Һ��Ӧ������������Һ��Ӧ��
�ٳ����£���W�ĵ��ʺͼ���Һ��ϣ�������Ӧ�����ӷ���ʽΪ��
��Q��W����Ԫ�ؽ����Ե�ǿ��ΪQ W���������������) �����б�����֤����һ��ʵ���� ��
a.Q�ĵ��ʵ��۵��W���ʵ�
b.Q������������ˮ����ļ��Ա�W������������ˮ����ļ���ǿ
c. W��ԭ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и������ʵĹ�ϵ������ȷ����
A��35Cl��37Cl��ͬ�������� B��12C��13C��14C��ͬ�ֺ���
C��O2��O3��ͬλ�� D��H2O��D2O��ѧ������ͬ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com