

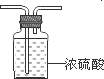
 ��
�� ��
��| 0.528g |
| 44g/mol |
| 0.144g |
| 0.352g |
| 0.144g |
| 18g/mol |
| 0.016g |
| 0.352g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
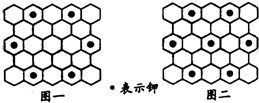 ������ѧ���й���ѧԺ�Ļ�ѧ�����ߺ������ѳɹ����Ƴ��������C60�γɵ�ʯī�в����ӻ������ʯī�������ڵļػ���̬�ļ��У�ʯī���ռض��γɳ�Ϊ��ʯī�����ʣ�����ɿ�����C8K��C12K��C24K��C36K��C48K��C60K�ȵȣ��ڼ�ʯī�У���ԭ�ӰѼ۵��ӽ���ʯī�㣬�����������������Ӧ����������ʱ�����ջأ����з�������ȷ���ǣ�������
������ѧ���й���ѧԺ�Ļ�ѧ�����ߺ������ѳɹ����Ƴ��������C60�γɵ�ʯī�в����ӻ������ʯī�������ڵļػ���̬�ļ��У�ʯī���ռض��γɳ�Ϊ��ʯī�����ʣ�����ɿ�����C8K��C12K��C24K��C36K��C48K��C60K�ȵȣ��ڼ�ʯī�У���ԭ�ӰѼ۵��ӽ���ʯī�㣬�����������������Ӧ����������ʱ�����ջأ����з�������ȷ���ǣ�������| A����������ٳ���6�ּ�ʯī������ͬ�������� | ||
| B����ij��ʯī��ԭ�ڷֲ���ͼһ��ʾ����������ʾ����C24K | ||
| C����ij��ʯī��ԭ�ӷֲ���ͼ����ʾ����������ʾ����C12K | ||
D������һ�ֻ�ɫ�ļ�ʯīC32K������K�ķֲ�Ҳ����ͼ�е����������Σ����������Kԭ��֮��ľ���Ϊʯī������4
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Ԫ�صĵ縺��Խ��Ԫ��ԭ�ӵĵ�һ������һ��Խ�� |
| B����Ԫ�����ڱ��У�Ԫ�ص縺�Դ�����Խ��ԽС |
| C������Ԫ�صĵ縺��һ��С�ڷǽ���Ԫ�صĵ縺�� |
| D�����γɻ�����ʱ���縺��ԽС��Ԫ��Խ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2 mol A��1 mol B |
| B��1 mol A��1 mol B |
| C��1 mol A��2 mol B |
| D��1 mol B��1 mol C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Na2SO3�Ѳ��ֱ����� |
| B������Ba��NO3��2��Һ�����ɵij�����һ������BaSO4 |
| C���������IJ��ܳ���һ����BaSO4��BaSO3 |
| D����ʵ�鲻��ȷ��Na2SO3�Ƿֱ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڢ� | B���ڢ� | C���٢� | D��ֻ�Т� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | �� | |
| 4 | �� | ? | ? |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ķǽ����Ա�̼ǿ��ֻ���ڸ����²��ܸ������Ϸ�Ӧ |
| B�����ǹ��ɿ������ʯ����ҪԪ�أ����ڵؿ��еĺ��������е�Ԫ���оӵ�һλ |
| C����Ļ�ѧ���ʲ����ã�����Ȼ���п���������̬���� |
| D�����ڵ��ӹ�ҵ������Ҫ�İ뵼����� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com