
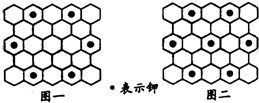
 ������ѧ���й���ѧԺ�Ļ�ѧ�����ߺ������ѳɹ����Ƴ��������C60�γɵ�ʯī�в����ӻ������ʯī�������ڵļػ���̬�ļ��У�ʯī���ռض��γɳ�Ϊ��ʯī�����ʣ�����ɿ�����C8K��C12K��C24K��C36K��C48K��C60K�ȵȣ��ڼ�ʯī�У���ԭ�ӰѼ۵��ӽ���ʯī�㣬�����������������Ӧ����������ʱ�����ջأ����з�������ȷ���ǣ�������
������ѧ���й���ѧԺ�Ļ�ѧ�����ߺ������ѳɹ����Ƴ��������C60�γɵ�ʯī�в����ӻ������ʯī�������ڵļػ���̬�ļ��У�ʯī���ռض��γɳ�Ϊ��ʯī�����ʣ�����ɿ�����C8K��C12K��C24K��C36K��C48K��C60K�ȵȣ��ڼ�ʯī�У���ԭ�ӰѼ۵��ӽ���ʯī�㣬�����������������Ӧ����������ʱ�����ջأ����з�������ȷ���ǣ�������| A����������ٳ���6�ּ�ʯī������ͬ�������� | ||
| B����ij��ʯī��ԭ�ڷֲ���ͼһ��ʾ����������ʾ����C24K | ||
| C����ij��ʯī��ԭ�ӷֲ���ͼ����ʾ����������ʾ����C12K | ||
D������һ�ֻ�ɫ�ļ�ʯīC32K������K�ķֲ�Ҳ����ͼ�е����������Σ����������Kԭ��֮��ľ���Ϊʯī������4
|
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
| 3 |
| 3 |


 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Һ��ˮ�����c��OH-����pH=4��NH4Cl��Һ��ˮ�����c��OH-����ͬ |
| B������Һ1mLϡ����100mL��pH����6 |
| C�������Һ�м���������pH=10������������Һǡ����ȫ�к� |
| D������Һ��п��Ӧ��pH������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1 mol��������2NA����ԭ�� |
| B��ʹ��Ħ��ʱ����ָ�����ӵ����� |
| C��1 mol�κ�����������ԭ����ΪNA�� |
| D��1 mol�κ�����������������ΪNA�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(CH3COO-) |
| c(H+) |
| c(CH3COO-) |
| c(CH3COOH)?c(OH-) |
| ��Һ | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ������Ϊ1��6��7��Ԫ�� |
| B��O��S��P |
| C��Fe��Cu��Cl |
| D��Na��Li��Mg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
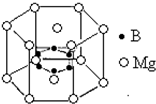
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��һ���ǹ��ۼ� |
| B��һ�������Ӽ� |
| C�������ǹ��ۼ���Ҳ���������Ӽ� |
| D��һ���Ǽ��Թ��ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com