
| c(H+)c(OH-) |
| c(H2O) |
| 1000g |
| 18g/mol |
| ||
| 18g/mol |
| c(H+)c(OH-) |
| c(H2O) |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
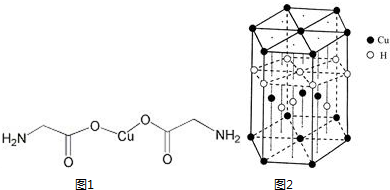 �-������ͭ��������Ļ���������Cu4O��PO4��2����ͨ�����з�Ӧ�Ʊ���2Na3PO4+4CuSO4+2NH3?H2O=Cu4O��PO4��2��+3Na2SO4+��NH4��2SO4+H2O
�-������ͭ��������Ļ���������Cu4O��PO4��2����ͨ�����з�Ӧ�Ʊ���2Na3PO4+4CuSO4+2NH3?H2O=Cu4O��PO4��2��+3Na2SO4+��NH4��2SO4+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ᱣ�������ϸǵIJ���ƿ�� |
| B����ˮʢ������ɫϸ��ƿ�� |
| C��Һ��ʢ��������Ƥ���IJ���ƿ�� |
| D����̬�������ɫ��ϸ��ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A����ϵͳ��������������  ��������2��3��5��5-�ļ�-4��4-���һ����� ��������2��3��5��5-�ļ�-4��4-���һ����� |
| B������������ϩ��2-��-2-��ϩ��ȫȼ������������������� |
| C������ʽΪC10H20��ϩ�������е�10��Cԭ�ӿ�����һ��ƽ���� |
| D������ʽΪC8H18�ķ����е�����Cԭ�Ӳ����ܶ���һ��ƽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| A���٢ܱ��ֳ����� |
| B���٢ڱ��ֳ������� |
| C���ۼȱ��ֳ��������ֱ��ֳ����� |
| D���ܱۢ��ֳ���ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ˮ�ĵ���̶���С������ˮ�ĵ���̶���� |
| B�����٢ܻ�ϣ���c��CH3COO-����c��H+��������Һһ���ʼ��� |
| C�����ķ���Һ�ֱ�ϡ�͵�ԭ�����ͬ��������Һ��pH���ۣ��ܣ��ڣ��� |
| D�����ڢۻ�ϣ���pH=7����������Һ��������ڣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��C��s��+
| ||
| B��2H2��g��+O2��g���T2H2O��g������H=+571.6 kJ?mol-1 | ||
| C��CH4��g��+2O2��g���TCO2��g��+2H2O��g������H=-890.3 kJ?mol-1 | ||
D��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��10mL 0.2mol/L��FeCl3��Һ |
| B��30mL 0.25mol/L��FeCl2��Һ |
| C��20mL 0.2mol/L��KCl��Һ |
| D��10mL 0.3mol/L��AlCl3��Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com