
 ��������ˮFeCl3���۵�Ϊ555K���е�Ϊ588K����ҵ���Ʊ���ˮFeCl3��һ�ֹ������£�
��������ˮFeCl3���۵�Ϊ555K���е�Ϊ588K����ҵ���Ʊ���ˮFeCl3��һ�ֹ������£�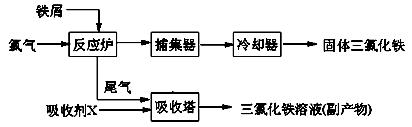
| �¶�/�� | 0 | 10 | 20 | 30 | 50 | 80 | 100 |
| �ܽ��(g/100gH20) | 74.4 | 81.9 | 91.8 | 106.8 | 315.1 | 525.8 | 535.7 |
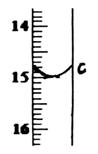
| �ζ����� | ����Һ���(mL) | �ζ�ǰ����(mL) | �ζ������(mL) |
| ��һ�� | 25.00 | 0.00 | c= |
| �ڶ��� | 25.00 | 0.00 | 14.99 |
| ������ | 25.00 | 0.00 | 15.01 |
 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A������ | B������ | C��ˮ���� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�������ƾ� | B�����Ȼ�̼���� | C�����͡����� | D�����͡���ˮ |

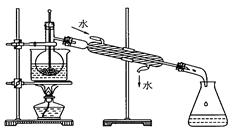
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
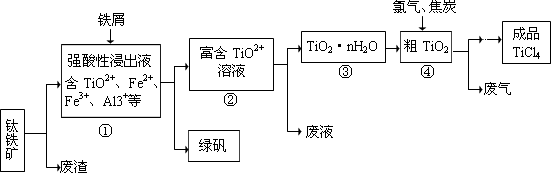
| �� | TiCl4 | SiCl4 |
| �۵�/�� | ��25.0 | ��68.8 |
| �е�/�� | 136.4 | 57.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

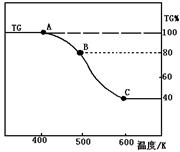
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��狀�KClΪԭ�Ͽ��Ժϳ�����ҪӦ�ü�ֵ���ơ�����ء���������淋����ʡ��ϳ�·�����£���
��狀�KClΪԭ�Ͽ��Ժϳ�����ҪӦ�ü�ֵ���ơ�����ء���������淋����ʡ��ϳ�·�����£���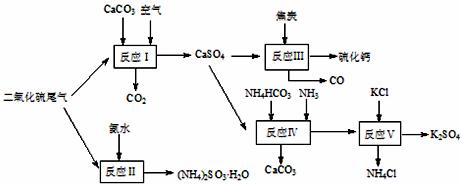
 �м��������ĶԱ����ӵ����ʣ���Ŀ���� �� ��
�м��������ĶԱ����ӵ����ʣ���Ŀ���� �� ��| A����Ӧ��������������������Ա�֤����������������������� |
B����Ӧ���з�����Ӧ�Ļ�ѧ����ʽΪ��CaSO4+4C CaS +4CO�� CaS +4CO�� |
| C����Ӧ���������60��70�棬Ŀ��֮һ�Ǽ���̼����淋ķֽ� |
| D����Ӧ���еĸ������Ȼ�刺��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
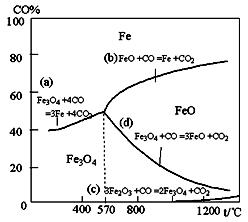
| ��Ӧ��� | ��ѧ��Ӧ | ��Ӧ�� |
| �� | Fe2O3(s)��3CO(g)=2Fe(s)��3CO 2(g) | ��H1= -26.7kJ��mol-1 |
| �� | 3Fe2O3(s)��CO(g)=2Fe3O4(s)��CO2(g) | ��H2= -50.8kJ��mol-1 |
| �� | Fe3O4(s)��CO(g)=3FeO(s)��CO2 (g) | ��H3= -36.5kJ��mol-1 |
| �� | FeO(s)��CO(g)=Fe(s)��CO2(g) | ��H4 |
 Fe(s)��CO2(g)��ƽ�ⳣ��K=0.4������һ�ܱ������У�����7.2gFeO��ͬʱͨ��4.48LCO(���ۺ�Ϊ��״��)���������µ�1100�棬��ά���¶Ȳ��䣬��ƽ��ʱ��FeO��ת����Ϊ�� ��
Fe(s)��CO2(g)��ƽ�ⳣ��K=0.4������һ�ܱ������У�����7.2gFeO��ͬʱͨ��4.48LCO(���ۺ�Ϊ��״��)���������µ�1100�棬��ά���¶Ȳ��䣬��ƽ��ʱ��FeO��ת����Ϊ�� ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com