
ЁОЬтФПЁПФПЧАЃЌШМУКЭбСђЪЧПЦбаЙЄзїепбаОПЕФживЊПЮЬтжЎвЛЃЌжївЊЭбСђЗНЗЈгавдЯТМИжжЃК
ЂёЃЎЪЏЛвЗЈдРэЮЊЃК2CaO(s)+2SO2(g)+O2(g)2CaSO4(s)
ЃЈ1ЃЉT1ЁцЪБЃЌЯђ10LКуШнУмБеШнЦїжаМгШы3molCaO(s)ЃЌВЂЭЈШы2molSO2(g)КЭ1molO2(g)ЗЂЩњЩЯЪіЗДгІЃЌ2minЪБДяЦНКтЃЌДЫЪБCaSO4(s)ЮЊ1.8molЁЃ0~2minФкЃЌгУSO2(g)БэЪОЕФИУЗДгІЕФЫйТЪv(SO2)=__________________________ЁЃ
ЂђЃЎДпЛЏбѕЛЏЗЈ
ЛюадЬПДпЛЏбѕЛЏЗЈЪЧЛљгкЛюадСМКУЕФЮяРэЮќИНКЭЛЏбЇЮќИНзїгУЃЌЦфЗДгІЛњРэЮЊЃК
O2+2CЁњ2CЁЊOЃЛSO2+CЁњCЁЊSO2ЃЛCЁЊSO2+CЁЊOЁњCЁЊSO3+CЃЛ
CЁЊSO3+H2OЁњCЁЊH2SO4ЃЛCЁЊH2SO4ЁњH2SO4+CЁЃ
ЃЈ2ЃЉећИіЙ§ГЬжазмЕФЛЏбЇЗДгІЗНГЬЪНЮЊ__________________________________________ЁЃ
ЃЈ3ЃЉЛюадЬПдкЗДгІЙ§ГЬжазїДпЛЏМСЃЌИФБфСЫ_____________ЃЈЬюбЁЯюзжФИЃЉЁЃ
AЃЎЗДгІЫйТЪ BЃЎЗДгІЯоЖШ CЃЎЗДгІьЪБф DЃЎЗДгІТЗОЖ EЃЎЗДгІЛюЛЏФм
ЂѓЃЎCOЛЙдЗЈдРэЮЊ2CO(g)+SO2(g)S(g)+2CO2(g) ІЄH
вбжЊЃКS(g)+O2(g)=SO2(g) ІЄH1=-574.0kJЁЄmol-1ЃЛCOШМЩеШШЮЊ283.0kJЁЄmol-1ЁЃ
ЃЈ4ЃЉЦ№ЪМЮТЖШЮЊT2ЁцЪБЃЌЗжБ№дкШ§ИіШнЛ§ОљЮЊ10LЕФОјШШУмБеШнЦїжаЃЌЗЂЩњЗДгІ2CO(g)+SO2(g)S(g)+2CO2(g)ЃЌВтЕУЯрЙиЪ§ОнШчЯТБэЫљЪОЁЃ
ШнЦї | Ц№ЪМЪБЮяжЪЕФСП/mol | ЦНКтЪБCO2(g)ЕФЮяжЪЕФСП/mol | |||
CO(g) | SO2(g) | S(g) | CO2(g) | ||
Мз | 1 | 0.5 | 0.5 | 0 | A |
вв | 1 | 0.5 | 0 | 0 | 0.8 |
Бћ | 2 | 1 | 0 | 0 | b |
ЂйШнЦїввДяЕНЦНКтКѓЃЌБЃГжЦфЫћЬѕМўВЛБфЃЌдйЯђЦфжаГфШыCO(g)ЁЂ SO2(g)ЁЂS(g)ЁЂCO2(g)Иї1molЃЌДЫЪБv(е§)__________ v(Фц)ЁЃЃЈЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁА=ЁБ)ЁЃ
ЂкЯТСаХаЖЯе§ШЗЕФЪЧ________________ЃЈЬюбЁЯюзжФИЃЉЁЃ
AЃЎb=1.6 BЃЎЦНКтГЃЪ§МзЃОвв CЃЎДяЕНЦНКтЕФЪБМфЃКБћЃМвв
ЁОД№АИЁП0.09molЁЄL-1ЁЄmin-1 2SO2 + O2 + 2H2O = 2H2SO4 AЁЂDЁЂE ЃО BC
ЁОНтЮіЁП
IЁЂ(1)ИљОнЗДгІ2CaO(s)+2SO2(g)+O2(g)2CaSO4(s)ПЩжЊЩњГЩ1.8molCaSO4ЃЌвЊЯћКФ1.8mol SO2ЃЌдђ2minФкSO2ХЈЖШБфЛЏСП=![]() =0.18mol/LЃЌгУSO2(g)БэЪОЕФИУЗДгІЫйТЪ=
=0.18mol/LЃЌгУSO2(g)БэЪОЕФИУЗДгІЫйТЪ=![]() =0.09mol/(LЁЄmin)ЁЃ
=0.09mol/(LЁЄmin)ЁЃ
IIЁЂ(2)вбжЊЂйO2 +2CЁњ2CЁЊOЃЛЂкSO2 +CЁњCЁЊSO2ЃЛЂлCЁЊSO2 + CЁЊOЁњCЁЊSO3 + CЃЛЂмCЁЊSO3+H2OЁњ CЁЊH2SO4ЃЛЂнCЁЊH2SO4ЁњH2SO4 + CЁЃНЋ[Ђй![]() +Ђк+Ђл+Ђм+Ђн]
+Ђк+Ђл+Ђм+Ђн]![]() 2ЕУ2SO2 + O2 + 2H2O
2ЕУ2SO2 + O2 + 2H2O![]() 2H2SO4ЁЃ
2H2SO4ЁЃ
(3)ДпЛЏМСФмЙЛМѕаЁЗДгІЕФЛюЛЏФмЃЌДгЖјИФБфЗДгІЫйТЪЃЌДпЛЏМСВЮгыЗДгІЖјИФБфЗДгІТЗОЖЃЌгЩДЫНтД№ЁЃ
IIIЁЂ(4)вбжЊЃКCOШМЩеШШЮЊ283.0 kJЁЄmol-1ЃЌгаШШЛЏбЇЗНГЬЪНЃКЂй2CO(g)+O2(g)=2CO2(g), ІЄH=-566.0kJ/molЃЛЂкS(g)+O2(g)=SO2(g),ІЄH1=-574.0 kJЁЄmol-1ЃЌИљОнИЧЫЙЖЈТЩНЋЂйЃЂкЕУ2CO(g)+SO2(g)S(g)+2CO2(g) ,ІЄH=-566.0kJ/mol-(-574.0kJ/mol)=+8kJ/molЃЛМД2CO(g)+SO2(g)S(g)+2CO2(g) ,ІЄH=+8kJ/molЁЃЂйвђЮЊШнЦїввЮТЖШВЛБфЃЌЫљвдЦНКтГЃЪ§ВЛБфЃЌдйЯђШнЦїжаГфШыCO(g)ЁЂ SO2(g)ЁЂS(g)ЁЂCO2(g)Иї1molЃЌМЦЫуЦфХЈЖШЩЬQ=![]() дйгыЦНКтГЃЪ§БШНЯНтД№ЁЃЂкA.МйЖЈШнЦїввгыШнЦїБћЯрЭЌЮТЖШКЭЬхЛ§ЃЌвђШнЦїБћГѕЪМЮяжЪЕФСПЪЧШнЦїввГѕЪМЮяжЪЕФСПЕФ2БЖЃЌЙЪШнЦїБћЗДгІЯрЖдгкШнЦїввЗДгІЕШаЇгкМгбЙЃЌдйПМТЧЪЕМЪЪЧдкОјШШЬѕМўЯТЃЌБШНЯШнЦїБћЕФЮТЖШгыШнЦїввЕФЮТЖШИпЕЭРДзїД№ЁЃB.МйЖЈШнЦїМзЁЂввДІгкКуЮТКуШнЯТЃЌДгБэИёГѕЪМЭЖСЯПДЃЌШнЦїМзДяЕНЕФЦНКтЯрЕБгкдкШнЦїввЦНКтжадйМгШы0.5molS(g)ЃЌШнЦїМзЦНКтЯђФцЗДгІЗНЯђвЦЖЏЃЌЙЪШнЦїМзЗДгІЮќЪеЕФШШСПБШШнЦїввЩйЃЌЕЋШнЦїМзЁЂввЪЕМЪДІгкОјШШЬѕМўЯТЃЌдйБШНЯШнЦїМзгыШнЦїввЕФЮТЖШИпЕЭЃЌИљОнЦНКтГЃЪ§гыЮТЖШгаЙиЗжЮіЁЃC.МйЖЈдкКуЮТКуШнЯТЃЌввЁЂБћСНШнЦїДяЕНЕФЦНКтЛЅЮЊЕШаЇЦНКтЃЌШнЦїБћЮќЪеЕФШШСПЪЧШнЦїввЕФ2БЖЃЌШнЦїБћжаИїзщЗжЕФХЈЖШЪЧШнЦїввжаЖдгІзщЗжХЈЖШЕФ2БЖЃЌЧвШнЦїБћБШШнЦїввЖрЮќЪеЕФШШСП=
дйгыЦНКтГЃЪ§БШНЯНтД№ЁЃЂкA.МйЖЈШнЦїввгыШнЦїБћЯрЭЌЮТЖШКЭЬхЛ§ЃЌвђШнЦїБћГѕЪМЮяжЪЕФСПЪЧШнЦїввГѕЪМЮяжЪЕФСПЕФ2БЖЃЌЙЪШнЦїБћЗДгІЯрЖдгкШнЦїввЗДгІЕШаЇгкМгбЙЃЌдйПМТЧЪЕМЪЪЧдкОјШШЬѕМўЯТЃЌБШНЯШнЦїБћЕФЮТЖШгыШнЦїввЕФЮТЖШИпЕЭРДзїД№ЁЃB.МйЖЈШнЦїМзЁЂввДІгкКуЮТКуШнЯТЃЌДгБэИёГѕЪМЭЖСЯПДЃЌШнЦїМзДяЕНЕФЦНКтЯрЕБгкдкШнЦїввЦНКтжадйМгШы0.5molS(g)ЃЌШнЦїМзЦНКтЯђФцЗДгІЗНЯђвЦЖЏЃЌЙЪШнЦїМзЗДгІЮќЪеЕФШШСПБШШнЦїввЩйЃЌЕЋШнЦїМзЁЂввЪЕМЪДІгкОјШШЬѕМўЯТЃЌдйБШНЯШнЦїМзгыШнЦїввЕФЮТЖШИпЕЭЃЌИљОнЦНКтГЃЪ§гыЮТЖШгаЙиЗжЮіЁЃC.МйЖЈдкКуЮТКуШнЯТЃЌввЁЂБћСНШнЦїДяЕНЕФЦНКтЛЅЮЊЕШаЇЦНКтЃЌШнЦїБћЮќЪеЕФШШСПЪЧШнЦїввЕФ2БЖЃЌШнЦїБћжаИїзщЗжЕФХЈЖШЪЧШнЦїввжаЖдгІзщЗжХЈЖШЕФ2БЖЃЌЧвШнЦїБћБШШнЦїввЖрЮќЪеЕФШШСП=![]() ЁС8kJ/molЁС0.8mol=3.2kJЁЃЖјЪЕМЪЪЧдкОјШШЬѕМўЯТЃЌДЫЪБХЈЖШЖдЗДгІЫйТЪЕФгАЯьГЌЙ§СЫЮТЖШЖдЗДгІЫйТЪЕФгАЯьЃЌЙЪШнЦїБћЕФЗДгІЫйТЪБШШнЦїввЕФЗДгІЫйТЪДѓЃЌЗДгІЫйТЪдНДѓДяЕНЦНКтЕФЪБМфдНаЁЃЌгЩДЫЗжЮіЁЃ
ЁС8kJ/molЁС0.8mol=3.2kJЁЃЖјЪЕМЪЪЧдкОјШШЬѕМўЯТЃЌДЫЪБХЈЖШЖдЗДгІЫйТЪЕФгАЯьГЌЙ§СЫЮТЖШЖдЗДгІЫйТЪЕФгАЯьЃЌЙЪШнЦїБћЕФЗДгІЫйТЪБШШнЦїввЕФЗДгІЫйТЪДѓЃЌЗДгІЫйТЪдНДѓДяЕНЦНКтЕФЪБМфдНаЁЃЌгЩДЫЗжЮіЁЃ
IЁЂ(1)ИљОнЗДгІ2CaO(s)+2SO2(g)+O2(g)2CaSO4(s)ПЩжЊЩњГЩ1.8molCaSO4ЃЌвЊЯћКФ1.8mol SO2ЃЌдђ2minФкSO2ХЈЖШБфЛЏСП=![]() =0.18mol/LЃЌгУSO2(g)БэЪОЕФИУЗДгІЫйТЪ=
=0.18mol/LЃЌгУSO2(g)БэЪОЕФИУЗДгІЫйТЪ=![]() =0.09mol/(LЁЄmin)ЁЃ
=0.09mol/(LЁЄmin)ЁЃ
IIЁЂ(2)вбжЊЂйO2 +2CЁњ2CЁЊOЃЛЂкSO2 +CЁњCЁЊSO2ЃЛЂлCЁЊSO2 + CЁЊOЁњCЁЊSO3 + CЃЛЂмCЁЊSO3+H2OЁњ CЁЊH2SO4ЃЛЂнCЁЊH2SO4ЁњH2SO4 + CЁЃНЋ[Ђй![]() +Ђк+Ђл+Ђм+Ђн]
+Ђк+Ђл+Ђм+Ђн]![]() 2ЯћГ§жаМфЮяЃЌЕУ2SO2 + O2 + 2H2O
2ЯћГ§жаМфЮяЃЌЕУ2SO2 + O2 + 2H2O![]() 2H2SO4
2H2SO4![]() 2H2SO4ЁЃ
2H2SO4ЁЃ
(3)ДпЛЏМСФмЙЛМѕаЁЗДгІЕФЛюЛЏФмЃЌЬсИпЛюЛЏЗжзгАйЗжЪ§ЃЌДгЖјИФБфЗДгІЫйТЪЃЛДпЛЏМСВЮгыЛЏбЇЗДгІЖјИФБфЗДгІТЗОЖЃЛЕЋДпЛЏМСВЛгАЯьЦНКтвЦЖЏЃЌЫљвдВЛИФБфЗДгІЯоЖШЃЛДпЛЏМСВЛФмИФБфЗДгІЮяКЭЩњГЩЮяОпгаЕФФмСПЃЌЙЪВЛИФБфЗДгІьЪБфЃЌД№АИбЁADEЁЃ
IIIЁЂ(4)вбжЊCOШМЩеШШЮЊ283.0 kJЁЄmol-1ЃЌгаШШЛЏбЇЗНГЬЪНЃКЂй2CO(g)+O2(g)=2CO2(g), ІЄH=-566.0kJ/molЃЛЂкS(g)+O2(g)=SO2(g),ІЄH1=-574.0 kJЁЄmol-1ЃЌИљОнИЧЫЙЖЈТЩНЋЂйЃЂкЕУ2CO(g)+SO2(g)S(g)+2CO2(g),ІЄH=-566.0kJ/mol-(-574.0kJ/mol)=+8kJ/molЃЛМД2CO(g)+SO2(g)S(g)+2CO2(g) ,ІЄH=+8kJ/molЁЃ
ЂйИљОнБэИёЪ§ОнПЩжЊШнЦїввДяЕНЦНКтЪБЩњГЩ0.8molCO2ЃЌгЩЗНГЬЪН2CO(g)+SO2(g)S(g)+2CO2(g)МЦСПЙиЯЕМЦЫуЕУЦНКтЪБЃКS(g)ЮяжЪЕФСП= 0.4molЁЂSO2ЮяжЪЕФСП=0.5mol-0.8molЁС![]() =0.1molЁЂCOЕФЮяжЪЕФСП=1mol-0.8mol=0.2molЃЌДЫЮТЖШЯТЦНКтГЃЪ§K=
=0.1molЁЂCOЕФЮяжЪЕФСП=1mol-0.8mol=0.2molЃЌДЫЮТЖШЯТЦНКтГЃЪ§K=![]() =
=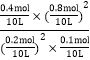 = 64ЁЃБЃГжЮТЖШВЛБфдйГфШыCO(g)ЁЂ SO2(g)ЁЂS(g)ЁЂCO2(g)Иї1molЃЌЦфХЈЖШЩЬQ=
= 64ЁЃБЃГжЮТЖШВЛБфдйГфШыCO(g)ЁЂ SO2(g)ЁЂS(g)ЁЂCO2(g)Иї1molЃЌЦфХЈЖШЩЬQ=![]() =
=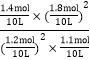 =
=![]() <K=64ЃЌЫЕУїЦНКтЯђе§ЗДгІЗНЯђНјааЃЌМДДЫЪБv(е§)>v(Фц)ЁЃ
<K=64ЃЌЫЕУїЦНКтЯђе§ЗДгІЗНЯђНјааЃЌМДДЫЪБv(е§)>v(Фц)ЁЃ
ЂкA.МйЖЈШнЦїввгыШнЦїБћЯрЭЌЮТЖШКЭЬхЛ§ЃЌвђШнЦїБћГѕЪМЮяжЪЕФСПЪЧШнЦїввГѕЪМЮяжЪЕФСПЕФ2БЖЃЌЙЪШнЦїБћЗДгІЯрЖдгкШнЦїввЗДгІЕШаЇгкМгбЙЃЌЖјЗДгІ2CO(g)+SO2(g)S(g)+2CO2(g) ,ІЄH=+8kJ/molЧАКѓЦјЬхЗжзгЪ§ФПВЛБфЃЌИУЗДгІМгбЙЦНКтВЛвЦЖЏЃЌЙЪЦНКтЪБШнЦїБћжаCO2ЮяжЪЕФСПЪЧШнЦїввжаCO2ЮяжЪЕФСПЕФ2БЖМДЮЊ1.6molЃЌЮќЪеЕФШШСПвВЪЧШнЦїввЕФ2БЖЁЃЕЋЪЧЃЌЪЕМЪЪЧОјШШЬѕМўЯТЃЌШнЦїгыЛЗОГВЛЗЂЩњШШСПНЛЛЛЃЌШнЦїБћЕФЮТЖШБШШнЦїввЕЭЃЌНЕЮТИУЗДгІЯђФцЗДгІЗНЯђвЦЖЏЃЌЫљвдШнЦїБћжаЩњГЩЮяCO2ЕФСПНЋвЊМѕЩйЃЌЫљвдb<1.6ЃЌAЯюДэЮѓЃЛB.МйЖЈШнЦїМзЁЂввДІгкКуЮТКуШнЯТЃЌДгБэИёГѕЪМЭЖСЯПДЃЌШнЦїМзДяЕНЕФЦНКтЯрЕБгкдкШнЦїввЦНКтжадйМгШы0.5molS(g)ЃЌШнЦїМзЦНКтЯђФцЗДгІЗНЯђвЦЖЏЃЌЙЪШнЦїМзЗДгІЮќЪеЕФШШСПБШШнЦїввЩйЃЌЕЋЪЧЃЌШнЦїМзЁЂввЪЕМЪДІгкОјШШЬѕМўЯТЃЌЙЪШнЦїМзЮТЖШБШШнЦїввЮТЖШвЊИпЃЌЦНКтГЃЪ§гыЮТЖШгаЙиЃЌгжИУЗДгІе§ЗДгІЮЊЮќШШЗДгІЃЌЩ§ЮТЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌЫљвдЦНКтГЃЪ§Мз>ввЃЌBЯюе§ШЗЃЛC.ИљОнAЯюЗжЮіПЩжЊМйЖЈдкКуЮТКуШнЯТЃЌввЁЂБћСНШнЦїДяЕНЕФЦНКтЛЅЮЊЕШаЇЦНКтЃЌШнЦїБћЮќЪеЕФШШСПЪЧШнЦїввЕФ2БЖЃЌШнЦїБћжаИїзщЗжЕФХЈЖШЪЧШнЦїввжаЖдгІзщЗжХЈЖШЕФ2БЖЃЌЧвШнЦїБћБШШнЦїввЖрЮќЪеЕФШШСП=![]() ЁС8kJ/molЁС0.8mol=3.2kJЁЃЖјЪЕМЪЪЧдкОјШШЬѕМўЯТЃЌШнЦїБћЖрЮќЪеЕФ3.2kJШШСПЖдШнЦїБћЮТЖШНЕЕЭВЛДѓЃЌЖјШнЦїБћжаИїзщЗжЕФХЈЖШЪЧШнЦїввЕФ2БЖЃЌДЫЪБХЈЖШЖдЗДгІЫйТЪЕФгАЯьГЌЙ§СЫЮТЖШЖдЗДгІЫйТЪЕФгАЯьЃЌЙЪШнЦїБћЕФЗДгІЫйТЪБШШнЦїввЕФЗДгІЫйТЪДѓЃЌДяЕНЦНКтЕФЪБМфБћ<ввЃЌCЯюе§ШЗЃЛД№АИбЁBCЁЃ
ЁС8kJ/molЁС0.8mol=3.2kJЁЃЖјЪЕМЪЪЧдкОјШШЬѕМўЯТЃЌШнЦїБћЖрЮќЪеЕФ3.2kJШШСПЖдШнЦїБћЮТЖШНЕЕЭВЛДѓЃЌЖјШнЦїБћжаИїзщЗжЕФХЈЖШЪЧШнЦїввЕФ2БЖЃЌДЫЪБХЈЖШЖдЗДгІЫйТЪЕФгАЯьГЌЙ§СЫЮТЖШЖдЗДгІЫйТЪЕФгАЯьЃЌЙЪШнЦїБћЕФЗДгІЫйТЪБШШнЦїввЕФЗДгІЫйТЪДѓЃЌДяЕНЦНКтЕФЪБМфБћ<ввЃЌCЯюе§ШЗЃЛД№АИбЁBCЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПВнЫсбЧЬњОЇЬх(FeC2O4ЁЄxH2O)ЮЊЕЛЦЩЋЗлФЉЃЌВЛШмгкЫЎЃЌПЩзїееЯрЯдгАМСКЭгУгкжЦвЉЙЄвЕЁЃФГЛЏбЇаЫШЄаЁзщЖдЦфаджЪНјааШчЯТЬНОПЃЌЛиД№ЯТСаЮЪЬтЃК
I.ЖЈадЬНОП
бЁгУЯТСаЪдМСЩшМЦЪЕбщЗНАИЃЌЭъГЩЯТБэФкШнЁЃ
ЪдМСЃКЫсадKMnO4ШмвКЁЂK3[Fe(CN)6]ШмвК
Вйзї | ЯжЯѓ | НсТлгыНтЪЭ |
(1)ШЁЩйСПВнЫсбЧЬњОЇЬхгкЪдЙмжаЃЌМгШы2mLЫЎЃЌеёЕДКѓОВжУ | гаЕЛЦЩЋГСЕэЃЌЩЯВуЧхвКЮоЩЋ | ВнЫсбЧЬњВЛШмгкЫЎ |
(2)МЬајМгШы2mLЯЁСђЫсЃЌеёЕД | ___________ | ВнЫсбЧЬњШмгкСђЫсЃЌСђЫсЫсадЧПгкВнЫс |
(3)ЯђВНжш(2)ЫљЕУШмвКжаЕЮМгМИЕЮK3[Fe(CN)6]ШмвК | ВњЩњРЖЩЋГСЕэ | ___________ |
(4)Ђй___________ | Ђк___________ | H2C2O4ЛђC2O42ЃОпгаЛЙдад |
Ђђ.ЖЈСПЬНОПЃКЕЮЖЈЪЕбщВтxЕФжЕ
(5)ЕЮЖЈЧАЃЌЯТСаВйзїЕФе§ШЗЫГађЪЧ___________(ЬюзжФИађКХ)ЁЃ
a.гУ0.1000mol/LЕФЫсадKMnO4ШмвКШѓЯД
b.ВщТЉЁЂЧхЯД
c.ХХОЁЕЮЖЈЙмМтзьЕФЦјХнВЂЕїећвКУц
d.ЪЂзА0.1000mol/LЕФЫсадKMnO4ШмвК
e.ГѕЪМЖСЪ§ЁЂМЧТМЮЊ0.50mL
(6)ГЦШЁngбљЦЗЃЌМгШыЪЪСПЯЁСђЫсШмНтЃЌгУВНжш(5)зМБИЕФБъзМKMnO4ШмвКжБНгЕЮЖЈЃЌШчКЮХаЖЯЕЮЖЈжеЕу?_______________ЁЃ
(7)жеЕуЖСЪ§ЮЊ20.50mLЁЃНсКЯЩЯЪіЪЕбщЪ§ОнЧѓЕУx=___________(гУКЌnЕФДњЪ§ЪНБэЪОЃЌFeC2O4ЕФЯрЖдЗжзгжЪСПЮЊ144)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПКЯГЩАБЪЧШЫРрПЦбЇММЪѕЩЯЕФвЛЯюжиДѓЭЛЦЦЃЌЗДгІдРэЮЊN2(g)ЃЋ3H2(g)![]() 2NH3(g) ІЄHЃНЃ92.4 kJЁЄmolЃ1ЁЃвЛжжЙЄвЕКЯГЩАБЕФМђЪНСїГЬЭМШчЯТЃК
2NH3(g) ІЄHЃНЃ92.4 kJЁЄmolЃ1ЁЃвЛжжЙЄвЕКЯГЩАБЕФМђЪНСїГЬЭМШчЯТЃК

ЃЈ1ЃЉЂйВНжшЂђжажЦЧтЦјЕФдРэШчЯТЃК
aЃЎCH4(g)ЃЋH2O(g)![]() CO(g)ЃЋ3H2(g)ЃЛK1
CO(g)ЃЋ3H2(g)ЃЛK1
bЃЎCO(g)ЃЋH2O(g)![]() CO2(g)ЃЋH2(g)ЃЛK2
CO2(g)ЃЋH2(g)ЃЛK2
дђЗДгІCH4(g)ЃЋ2H2O(g)![]() CO2(g)ЃЋ4H2(g)ЃЛK=_______________(гУКЌK1ЁЂK2ЕФДњЪ§ЪНБэЪО)ЁЃ
CO2(g)ЃЋ4H2(g)ЃЛK=_______________(гУКЌK1ЁЂK2ЕФДњЪ§ЪНБэЪО)ЁЃ
ЂкT1ЮТЖШЪБЃЌЖдгкЗДгІЃКCO(g)+ H2O(g)![]() CO2(g)+ H2(g)ЃЌЯђ2 LЕФКуШнУмБеШнЦїжаЭЈШывЛЖЈСПЕФCOКЭH2O(g)ЁЃШєДяЕНЦНКтКѓЃЌЗДгІЗХШШQ kJЃЌБЃГжЦфЫћЬѕМўВЛБфЃЌжЛЪЧЯђдЦНКтЬхЯЕжадйЭЈШы0.20 mol H2O(g)ЃЌдђЯТСаЫЕЗЈе§ШЗЕФЪЧ_____________ЁЃ
CO2(g)+ H2(g)ЃЌЯђ2 LЕФКуШнУмБеШнЦїжаЭЈШывЛЖЈСПЕФCOКЭH2O(g)ЁЃШєДяЕНЦНКтКѓЃЌЗДгІЗХШШQ kJЃЌБЃГжЦфЫћЬѕМўВЛБфЃЌжЛЪЧЯђдЦНКтЬхЯЕжадйЭЈШы0.20 mol H2O(g)ЃЌдђЯТСаЫЕЗЈе§ШЗЕФЪЧ_____________ЁЃ
AЃЎCOЕФзЊЛЏТЪНЋдіДѓ BЃЎДяЕНаТЦНКтЪБЕФЗДгІШШІЄH ЃО ЁЊQ
CЃЎЦјЬхЕФУмЖШНЋВЛБф DЃЎH2OЕФЬхЛ§ЗжЪ§діДѓ
ЃЈ2ЃЉНЋ3 molH2КЭ2 molN2ГфШыФГКуЮТКубЙШнЦїжаЃЌЗЂЩњКЯГЩАБЕФЗДгІЃК3H2(g) ЃЋN2(g) ![]() 2NH3(g)
2NH3(g)
ЂйДяЦНКтЪБNH3ЕФХЈЖШЮЊc molЁЄL-1ЁЃБЃГжЮТЖШВЛБфЃЌАДЯТСаХфБШЗжБ№ГфШыИУШнЦїЃЌЦНКтКѓNH3ЕФХЈЖШВЛЮЊc molЁЄL-1ЕФЪЧ_________ЁЃ
AЃЎ6 molH2 + 4 molN2
BЃЎ0.75 molH2 + 0.75 molN2 + 0.5 molNH3
CЃЎ3 molH2+ 1 molN2 + 2 mol NH3
Ђк ШєЪЧдкКуЮТКуШнЕФШнЦїжаЗДгІЃЌДяЕНЦНКтЪБNH3ЕФХЈЖШЮЊc1 molЁЄL-1ЃЌдђc________c1ЃЈЬюЁА<ЁБЁА>ЁБЛђЁА=ЁБЃЉЃЌдвђЪЧ________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПКЌгаЯрЭЌбѕдзгЪ§ЕФЖўбѕЛЏСђКЭШ§бѕЛЏСђЃЌЮяжЪЕФСПжЎБШЪЧ___ЃЌжЪСПжЎБШЪЧ___ЁЃАб4mol/LCuSO4КЭ2mol/LH2SO4ШмвКЕШЬхЛ§ЛьКЯЃЈМйЩшЛьКЯКѓШмвКЕФЬхЛ§ЕШгкЛьКЯЧАШмвКЕФЬхЛ§жЎКЭЃЉЃЌЧѓЛьКЯШмвКжаC(CuSO4)=_____mol/LЃЌC(SO42-)=___mol/LЁЃНЋ10КСЩ§2mol/LЕФСђЫсШмвКМгЫЎЯЁЪЭЕН0.5mol/LЃЌЦфЬхЛ§ЮЊ____КСЩ§ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЫљЪОЃЌШєзЖаЮЦПФкЪЧЫЎЃЌЗжвКТЉЖЗФкЕФвКЬхвВЪЧЫЎЃЌЯђЩеБФкЕЮМгЫЎЪБЃЌЗЂЯжUаЮЙмФквКУцзѓБпЕЭгкгвБпЃЌЛжИДЕНдЮТЖШКѓвКУцзѓБпгыгвБпЛљБОЯрЦНЃЌдђЩеБФкЕФЮяжЪЪЧЃЈ ЃЉ
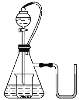
A.Й§бѕЛЏФЦB.бѕЛЏФЦC.ФЦD.ТШЛЏФЦ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдкЭЌЮТЭЌбЙЯТЃЌa gЦјЬхAгыb gЦјЬхBЕФЗжзгЪ§ЯрЭЌЃЌЯТСаЫЕЗЈжаВЛе§ШЗЕФЪЧ(ЁЁЁЁ)
A. AгыBСНжжЦјЬхЕФЯрЖдЗжзгжЪСПжЎБШЮЊaЁУb
B. дкЭЌЮТЭЌбЙЕФЬѕМўЯТЃЌAгыBСНжжЦјЬхЕФУмЖШжЎБШЮЊbЁУa
C. ЭЌжЪСПЕФAЁЂBСНжжЦјЬхЕФЗжзгИіЪ§жЎБШЮЊbЁУa
D. ЯрЭЌЬѕМўЯТЃЌЭЌЬхЛ§AЦјЬхгыBЦјЬхЕФжЪСПжЎБШЮЊaЁУb
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЮТЖШЪБЃЌN2гыH2ЗДгІЙ§ГЬжаЕФФмСПБфЛЏШчЭМЫљЪОЁЃЯТСаа№Ъіе§ШЗЕФЪЧ

A. е§ЗДгІЕФЛюЛЏФмЕШгкФцЗДгІЕФЛюЛЏФм
B. aЧњЯпЪЧМгШыДпЛЏМСЪБЕФФмСПБфЛЏЧњЯп
C. 1 mo N2гы3 mo H2ГфЗжЗДгІЗХГіЕФШШСПаЁгк92 kJ
D. МгШыДпЛЏМСЃЌИУЗДгІЕФьЪБфМѕаЁ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙлВьЪЧбаОПЮяжЪаджЪЕФвЛжжЛљБОЗНЗЈЁЃвЛЭЌбЇНЋвЛаЁПщН№ЪєФЦТЖжУгкПеЦјжаЃЌЙлВьЕНЯТСаЯжЯѓЃКвјАзЩЋ![]() БфЛвАЕ
БфЛвАЕ![]() БфАзЩЋ
БфАзЩЋ![]() ГіЯжвКЕЮ
ГіЯжвКЕЮ![]() АзЩЋЙЬЬхЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
АзЩЋЙЬЬхЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A.ЂйЗЂЩњСЫЛЏКЯЗДгІB.ЂкБфАзЩЋЪЧвђЮЊЩњГЩСЫЬМЫсФЦ
C.ЂлЪЧЬМЫсФЦЮќЪеПеЦјжаЕФЫЎеєЦјаЮГЩСЫШмвКD.ЂмжЛЗЂЩњЮяРэБфЛЏ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯћГ§КЌЕЊЛЏКЯЮяЖдДѓЦјКЭЫЎЬхЕФЮлШОЪЧЛЗОГБЃЛЄЕФживЊбаОППЮЬтЁЃ
(1)вбжЊЃКN2(g)ЃЋO2(g)===2NO(g)ЁЁЁЁЁЁЁЁІЄHЃНa kJЁЄmolЃ1
2NO(g)ЃЋO2(g)===2NO2(g)ЁЁ ІЄHЃНb kJЁЄmolЃ1
4NH3(g)ЃЋ5O2(g)===4NO(g)ЃЋ6H2O(l)ЁЁЁЁЁЁЁЁІЄHЃНc kJЁЄmolЃ1
ЗДгІ8NH3(g)ЃЋ6NO2(g)===7N2(g)ЃЋ12H2O(l)ЁЁ ІЄHЃН____kJЁЄmolЃ1ЁЃ
(2)ЫЎЬхжаЙ§СПАБЕЊ(вдNH3БэЪО)ЛсЕМжТЫЎЬхИЛгЊбјЛЏЁЃ
ЂйгУДЮТШЫсФЦГ§ШЅАБЕЊЕФдРэШчЭМ1ЫљЪОЁЃаДГіИУЭМЪОЕФзмЗДгІЛЏбЇЗНГЬЪНЃК______________________ЁЃИУЗДгІашПижЦЮТЖШЃЌЮТЖШЙ§ИпЪБАБЕЊШЅГ§ТЪНЕЕЭЕФдвђЪЧ________________________________________________________________________ЁЃ
 ЭМ1ЁЁЁЁ
ЭМ1ЁЁЁЁ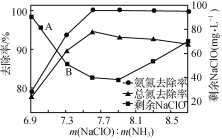 ЭМ2
ЭМ2

ЂкШЁвЛЖЈСПЕФКЌАБЕЊЗЯЫЎЃЌИФБфМгШыДЮТШЫсФЦЕФгУСПЃЌЗДгІвЛЖЮЪБМфКѓЃЌШмвКжаАБЕЊШЅГ§ТЪЁЂзмЕЊ(ШмвКжаЫљгаПЩШмадЕФКЌЕЊЛЏКЯЮяжаЕЊдЊЫиЕФзмСП)ШЅГ§ТЪвдМАЪЃгрДЮТШЫсФЦЕФКЌСПЫцm(NaClO)ЁУm(NH3)ЕФБфЛЏЧщПіШчЭМ2ЫљЪОЁЃЕуBЪЃгрNaClOКЌСПЕЭгкЕуAЕФдвђЪЧ____ЁЃЕБm(NaClO)ЁУm(NH3)>7.6ЪБЃЌЫЎЬхжазмЕЊШЅГ§ТЪЗДЖјЯТНЕЃЌПЩФмЕФдвђЪЧ____ЁЃ
(3)ЕчМЋЩњЮяФЄЕчНтЭбЯѕЪЧЕчЛЏбЇКЭЮЂЩњЮяЙЄвеЕФзщКЯЁЃФГЮЂЩњЮяФЄФмРћгУЕчНтВњЩњЕФЛюаддзгНЋNO3-ЛЙдЮЊN2ЃЌЙЄзїдРэШчЭМ3ЫљЪОЁЃ
ЂйаДГіИУЛюаддзггыNO3-ЗДгІЕФРызгЗНГЬЪНЃК________________ЁЃ
ЂкШєбєМЋЩњГЩБъзМзДПіЯТ2.24 LЦјЬхЃЌРэТлЩЯПЩГ§ШЅNO3-ЕФЮяжЪЕФСПЮЊ____molЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com