
| 11.65g |
| 233g/mol |
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CaO+H2O�TCa��OH��2 | ||||
| B��2Na2O2+2H2O�T4NaOH+O2�� | ||||
| C��2Na+2H2O�T2NaOH+H2�� | ||||
D��C+H2O
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
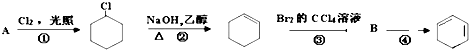
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ӻϳ������������壬���а���һ��ռ15%�������������Ĺ�ҵ��Ч�ʶ��ܵ� |
| B������NH3��Һ����N2��H2��ѭ��ʹ�ã����ܵ�˵�����IJ��ʺܸ� |
| C���ϳɰ���ҵ�ķ�Ӧ�¶ȿ�����400��500�����ң�����Ϊ�������°��IJ������ |
| D���ϳɰ���ҵ����10 MPa��30MPa������������´����Ļ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
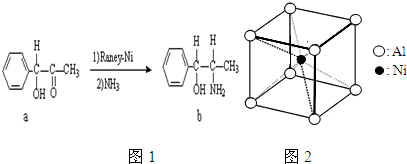
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 ��R��R��Ϊ�������⣩
��R��R��Ϊ�������⣩ +R2OH
+R2OH| һ������ |
 +HCl��R��R��������
+HCl��R��R��Ϊ������| NaOH��Һ |
| �� |
| CH3COOH |
| Ũ���ᣮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | I1 | I2 | I3 | I4 |
| X | 500 | 4 600 | 6 900 | 9 500 |
| Y | 580 | 1 800 | 2 700 | 11 600 |
| A��X�ĵ��ʳ���������ˮ���ҷ�Ӧ |
| B����Ԫ��Y���ڵ�3���ڣ�������NaOH��Һ��Ӧ |
| C��Ԫ��X�����γɻ�����ʱ����ѧʽΪXCl |
| D����Ԫ��Y���ڵ�3���ڣ��������γɵĻ�����Ϊ���ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ba2+��Na+��NH4+��Br-��Cl- |
| B��K+��Na+��AlO2-��SO42 -��SiO32 - |
| C��Na+��HCO3-��SO32 -��CH3COO- |
| D��H+��Fe3+��Na+��NO3-��SO32 - |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com