
ЎѕМвДїЎї¶юјЧГС(CH3OCH3)ЦШХыЦЖИЎH2Ј¬ѕЯУРОЮ¶ѕЎўОЮґМј¤РФµИУЕµгЎЈ»ШґрПВБРОКМвЈє
(1) CH3OCH3єНO2·ўЙъ·ґУ¦IЈєCH3OCH3(g)+1/2O2(g)=2CO(g)+3H2(g) ЎчH
ТСЦЄЈєCH3OCH3(g) ![]() CO(g)+H2(g)+CH4 (g) ЎчH1
CO(g)+H2(g)+CH4 (g) ЎчH1
CH4 (g)+3/2O2(g)=CO(g)+2H2O (g) ЎчH2
H2(g)+1/2O2(g)=H2O (g) ЎчH3
ўЩФт·ґУ¦IµДЎчH=____(УГє¬ЎчH1ЎўЎчH2ЎўЎчH3µДґъКэКЅ±нКѕ)ЎЈ
ўЪ±ЈіЦОВ¶ИєНС№ЗїІ»±дЈ¬·Ц±р°ґІ»Н¬ЅшБП±ИНЁИлCH3OCH3єНO2Ј¬·ўЙъ·ґУ¦IЎЈІвµГЖЅєвК±H2µДМе»э°Щ·Цє¬БїУлЅшБПЖшЦРn(O2)/n(CH3OCH3)µД№ШПµИзНјЛщКѕЎЈµ±n(O2)/n(CH3OCH3)Јѕ0.6К±Ј¬H2µДМе»э°Щ·Цє¬БїїмЛЩЅµµНЈ¬ЖдЦчТЄФТтКЗ____(Мо±кєЕ)ЎЈ

AЈ®№эБїµДO2ЖрПЎКНЧчУГ
BЈ®№эБїµДO2УлH2·ўЙъё±·ґУ¦ЙъіЙH2O
C Ј®n(O2)/n(CH3OCH3)Јѕ0.6ЖЅєвПтДж·ґУ¦·ЅПтТЖ¶Ї
(2)TЎжК±Ј¬ФЪєгИЭГЬ±ХИЭЖчЦРНЁИлCH3OCH3Ј¬·ўЙъ·ґУ¦IIЈєCH3OCH3(g)![]() CO(g)+H2(g)+CH4(g)Ј¬ІвµГИЭЖчДЪіхКјС№ЗїОЄ41.6 kPaЈ¬·ґУ¦№эіМЦР·ґУ¦ЛЩВКv(CH3OCH3)К±јдtУлCH3OCH3·ЦС№P(CH3OCH3)µД№ШПµИзНјЛщКѕЎЈ
CO(g)+H2(g)+CH4(g)Ј¬ІвµГИЭЖчДЪіхКјС№ЗїОЄ41.6 kPaЈ¬·ґУ¦№эіМЦР·ґУ¦ЛЩВКv(CH3OCH3)К±јдtУлCH3OCH3·ЦС№P(CH3OCH3)µД№ШПµИзНјЛщКѕЎЈ

ўЩt=400 sК±Ј¬CH3OCH3µДЧЄ»ЇВКОЄ____(±ЈБф2О»УРР§КэЧЦ)Ј»·ґУ¦ЛЩВКВъЧгv(CH3OCH3)=kPn(CH3OCH3),k=_____s-1Ј»400 sК±v(CH3OCH3)=_____kPaЈ®s-1ЎЈ
ўЪґпµЅЖЅєвК±Ј¬ІвµГМеПµµДЧЬС№ЗїPЧЬ= 121.6 kPaЈ¬ФтёГ·ґУ¦µДЖЅєвіЈКэKp=________________ kPa2(УГЖЅєв·ЦС№ґъМжЖЅєвЕЁ¶ИјЖЛгЈ¬·ЦС№=ЧЬС№ЎБОпЦКµДБї·ЦКэ)ЎЈ
ўЫёГОВ¶ИПВЈ¬ТЄЛх¶МґпµЅЖЅєвЛщРиµДК±јдЈ¬іэёДЅшґЯ»ЇјБНвЈ¬»№їЙІЙИЎµДґлК©КЗ____Ј¬ЖдАнУЙКЗ____ЎЈ
Ўѕґр°ёЎїЎчH1+ЎчH2-2ЎчH3 B 16% 4.4![]() 10-4 1.65
10-4 1.65![]() 10-2 4
10-2 4![]() 104 Фцґу·ґУ¦ОпµДС№Зї МбёЯ·ґУ¦ОпµДС№ЗїЈ¬»ЇС§·ґУ¦ЛЩВКјУїм
104 Фцґу·ґУ¦ОпµДС№Зї МбёЯ·ґУ¦ОпµДС№ЗїЈ¬»ЇС§·ґУ¦ЛЩВКјУїм
ЎѕЅвОцЎї
(1) ўЩёщѕЭёЗЛ№¶ЁВЙјЖЛгЈ»
ўЪУЙРЕПўїЙЦЄЈ¬№эБїµДO2УлH2·ўЙъё±·ґУ¦ЙъіЙH2OЈ¬К№H2µДМе»э°Щ·Цє¬БїїмЛЩЅµµНЈ»
(2)ўЩБРіцИэ¶ОКЅЈ¬ёщѕЭµИОВµИИЭМхјюПВЈ¬С№ЗїЦ®±ИµИУЪОпЦКµДБїЦ®±ИЈ¬ЗуіцЧЄ»ЇВКЈ»
ёщѕЭv(CH3OCH3)=kPn(CH3OCH3)єННјПуКэѕЭјЖЛгkЈ»ЅбєПНјПуКэѕЭЈ¬ёщѕЭЛЩВК№«КЅјЖЛгЛЩВКЈ»
ўЪБРіцИэ¶ОКЅЈ¬ХТіцЖЅєвК±CH3OCH3(g)ЎўCO(g)ЎўH2(g)ЎўCH4(g)·ЦС№Ј¬ФЩґъИлЖЅєвіЈКэ±нґпКЅјЖЛгЈ»
ўЫМбёЯ·ґУ¦ОпµДС№ЗїЈ¬ДЬјУїм»ЇС§·ґУ¦ЛЩВКЎЈ
(1) ўЩТСЦЄЈєi.CH3OCH3(g) ![]() CO(g)+H2(g)+CH4 (g) ЎчH1
CO(g)+H2(g)+CH4 (g) ЎчH1
ii.CH4 (g)+3/2O2(g)=CO(g)+2H2O (g) ЎчH2
iiiH2(g)+1/2O2(g)=H2O (g) ЎчH3
ёщѕЭёЗЛ№¶ЁВЙi+ii-iiiЎБ2µГЈєCH3OCH3(g)+1/2O2(g)=2CO(g)+3H2(g) ЎчH=ЎчH1+ЎчH2-2ЎчH3Ј¬
№Кґр°ёОЄЈєЎчH1+ЎчH2-2ЎчH3Ј»
ўЪ·ґУ¦IЈєCH3OCH3(g)+1/2O2(g) ![]() 2CO(g)+3H2(g)Ј¬УЙРЕПўїЙЦЄЈ¬№эБїµДO2УлH2·ўЙъё±·ґУ¦ЙъіЙH2OЈ¬К№H2µДМе»э°Щ·Цє¬БїїмЛЩЅµµНЈ¬AЎўCСЎПоІ»ДЬЛµГчH2µДМе»э°Щ·Цє¬БїїмЛЩЅµµНЈ¬№КСЎBЈ»
2CO(g)+3H2(g)Ј¬УЙРЕПўїЙЦЄЈ¬№эБїµДO2УлH2·ўЙъё±·ґУ¦ЙъіЙH2OЈ¬К№H2µДМе»э°Щ·Цє¬БїїмЛЩЅµµНЈ¬AЎўCСЎПоІ»ДЬЛµГчH2µДМе»э°Щ·Цє¬БїїмЛЩЅµµНЈ¬№КСЎBЈ»
№Кґр°ёОЄЈєBЈ»
(2)ўЩЙиЖрКјК±CH3OCH3µДОпЦКµДБїОЄnЈ¬Фт
CH3OCH3(g)![]() CO(g)+H2(g)+CH4(g)Ј¬
CO(g)+H2(g)+CH4(g)Ј¬
ЖрКјБїЈЁmolЈ© n 0 0 0
ЧЄ»ЇБїЈЁmolЈ© n![]() n
n![]() n
n![]() n
n![]()
400 sК±ЈЁmolЈ©n- n![]() n
n![]() n
n![]() n
n![]() ЧЬОпЦКµДБїЈєn+2n
ЧЬОпЦКµДБїЈєn+2n![]()
ёщѕЭµИОВµИИЭМхјюПВЈ¬С№ЗїЦ®±ИµИУЪОпЦКµДБїЦ®±ИЈ¬УР![]() =
=![]() Ј¬ЅвµГ
Ј¬ЅвµГ![]() =0.16=16%Ј»
=0.16=16%Ј»
УЙНјПуїЙЦЄЈ¬µ±P(CH3OCH3)=10.0kPaК±Ј¬v(CH3OCH3)=4.4ЎБ10-3kPaЎ¤s-1Ј¬
ёщѕЭv(CH3OCH3)=kPn(CH3OCH3)Ј¬n=1Ј¬Фтk=![]() s-1=4.4
s-1=4.4![]() 10-4 s-1Ј»
10-4 s-1Ј»
УЙНјПуїЙЦЄЈ¬400 sК±P(CH3OCH3)=35.0kPaЈ¬Фтv(CH3OCH3)=![]() =1.65ЎБ10-2kPaЈ®s-1ЎЈ
=1.65ЎБ10-2kPaЈ®s-1ЎЈ
№Кґр°ёОЄЈє16%Ј»4.4![]() 10-4Ј»1.65
10-4Ј»1.65![]() 10-2Ј»
10-2Ј»
ўЪґпµЅЖЅєвК±Ј¬ІвµГМеПµµДЧЬС№ЗїPЧЬ= 121.6 kPaЈ¬ЙиЖЅєвЧЄ»ЇВКОЄ![]() 1Ј¬Фт
1Ј¬Фт
CH3OCH3(g)![]() CO(g)+H2(g)+CH4(g)Ј¬
CO(g)+H2(g)+CH4(g)Ј¬
ЖрКјБїЈЁmolЈ© n 0 0 0
ЧЄ»ЇБїЈЁmolЈ© n![]() 1n
1n![]() 1n
1n![]() 1 n
1 n![]() 1
1
ЖЅєвБїЈЁmolЈ©n-n![]() 1n
1n![]() 1n
1n![]() 1 n
1 n![]() 1ЧЬОпЦКµДБїЈєn+2n
1ЧЬОпЦКµДБїЈєn+2n![]() 1
1
ёщѕЭµИОВµИИЭМхјюПВЈ¬С№ЗїЦ®±ИµИУЪОпЦКµДБїЦ®±ИЈ¬УР![]() =
=![]() Ј¬ЅвµГ
Ј¬ЅвµГ![]() 1=0.96Ј¬
1=0.96Ј¬
ФтЖЅєвК±CH3OCH3(g)ЎўCO(g)ЎўH2(g)ЎўCH4(g)·ЦС№·Ц±рОЄ1.67 kPaЎў39.98 kPaЎў39.98 kPaЎў39.98 kPaЈ¬ФтёГ·ґУ¦µДЖЅєвіЈКэKp=![]() 4
4![]() 104kPa2
104kPa2
ўЫіэК№УГґЯ»ЇјБНвЈ¬МбёЯ·ґУ¦ОпµДС№ЗїЈ¬ДЬјУїм»ЇС§·ґУ¦ЛЩВКЈ»
№Кґр°ёОЄЈє4![]() 104Ј»Фцґу·ґУ¦ОпµДС№ЗїЈ»МбёЯ·ґУ¦ОпµДС№ЗїЈ¬»ЇС§·ґУ¦ЛЩВКјУїмЎЈ
104Ј»Фцґу·ґУ¦ОпµДС№ЗїЈ»МбёЯ·ґУ¦ОпµДС№ЗїЈ¬»ЇС§·ґУ¦ЛЩВКјУїмЎЈ


 ФД¶БїміµПµБРґр°ё
ФД¶БїміµПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎї№вїМЅєКЗТ»ЦЦУ¦УГ№г·єµД№вГфІДБПЈ¬ЖдєПіЙВ·ПЯИзПВ(Ії·ЦКФјБЎў·ґУ¦МхјюєНІъОпТСВФИҐ)Јє

ТСЦЄЈєIЎў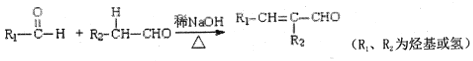
IIЎў
ЈЁ1Ј©A·ЦЧУµДГыіЖОЄ______Ј¬B·ЦЧУЦРЛщє¬№ЩДЬНЕµДГыіЖОЄ_______Ј¬УЙCµЅDµД·ґУ¦АаРНОЄ_______Ј»
ЈЁ2Ј©ТТИІєНфИЛбX·ўЙъјУіЙ·ґУ¦ЙъіЙEЈ¬EµДєЛґЕ№ІХсЗвЖЧУРИэЧй·еЈ¬ЗТ·еГж»э±ИОЄ3Јє2Јє1Ј¬EДЬ·ўЙъЛ®Ѕв·ґУ¦Ј¬ФтEµДЅб№№јтКЅОЄ_______Ј»
ЈЁ3Ј©DєНG·ґУ¦ЙъіЙ№вїМЅєµД»ЇС§·ЅіМКЅОЄ______Ј»
ЈЁ4Ј©CµДН¬·ЦТм№№МеВъЧгПВБРМхјюЈє
ўЩДЬ·ўЙъТшѕµ·ґУ¦Ј¬ЖдЛ®ЅвІъОпЦ®Т»ДЬУлFeCl3ИЬТє·ўЙъПФЙ«·ґУ¦Ј»
ўЪ·ЦЧУЦРЦ»УРТ»ёц»·ЧґЅб№№ЎЈВъЧгЙПКцМхјюµДН¬·ЦТм№№МеУР______ЦЦЎЈЖдЦР±Ѕ»·ЙПµДТ»ВИИЎґъІъОпЦ»УРБЅЦЦµДН¬·ЦТм№№МеµДЅб№№јтКЅОЄЈє_______
ЈЁ5Ј©ёщѕЭТСУРЦЄК¶ІўЅбєП±ѕМвРЕПўЈ¬РґіцТФ CH3CHOОЄФБПЦЖ±ёCH3COCOCOOHµДєПіЙВ·ПЯБчіМНј(ОЮ»ъКФјБИОУГЈ¬єПіЙВ·ПЯБчіМНјКѕАэјы±ѕМвМвёЙ)______ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїNiCO3іЈУГУЪґЯ»ЇјБЎўµз¶ЖЎўМХґЙµИ№¤ТµЎЈПЦУГДіє¬Дшµз¶Ж·ПФь(є¬CuЎўZnЎўFeЎўCrµИФУЦК)ЦЖИЎNiCO3µД№эіМИзНјЛщКѕЈє

ЈЁ1Ј©БчіМЦРµДКФјБXЈЁДіДЖСОЈ©µД»ЇС§КЅКЗ___________ЎЈ
ЈЁ2Ј©Ў°Сх»ЇЎ±К±Ри±ЈіЦВЛТєФЪ40ЎжЧуУТЈ¬УГ6%µДH2O2ИЬТєСх»ЇЎЈїШЦЖОВ¶ИІ»і¬№э40ЎжµДФТтКЗ______ЈЁУГ»ЇС§·ЅіМКЅ±нКѕЈ©ЎЈ
ЈЁ3Ј©Fe2+ТІїЙТФУГNaClO3Сх»ЇЈ¬ЙъіЙµДFe3+ФЪЅПРЎpHМхјюПВЛ®ЅвЈ¬ЧоЦХРОіЙ»ЖДЖМъ·Ї[Na2Fe6(SO4)4(OH)12]іБµн¶ш±»іэИҐЈ¬ИзНјКЗpHЎЄОВ¶И№ШПµНјЈ¬НјЦРТхУ°Ії·ЦОЄ»ЖДЖМъ·ЇОИ¶ЁґжФЪµДЗшУтЈ¬ПВБРЛµ·ЁІ»ХэИ·µДКЗ________(МоЧЦДё)ЎЈ

aЈ®»ЖДЖМъ·Ї[Na2Fe6(SO4)4(OH)12]ЦРМъОЄ+2јЫ
bЈ®pH№эµН»т№эёЯѕщІ»АыУЪЙъіЙ»ЖДЖМъ·ЇЈ¬ЖдФТтПаН¬
cЈ®ВИЛбДЖФЪСх»ЇFe2+К±Ј¬1 mol NaClO3µГµЅµДµзЧУКэОЄ6NA
dЈ®№¤ТµЙъІъЦРОВ¶ИіЈ±ЈіЦФЪ85Ў«95 ЎжЈ¬јУИлNa2SO4єуЙъіЙ»ЖДЖМъ·ЇЈ¬ґЛК±ИЬТєµДpHФјОЄ1.2Ў«1.8ЎЈ
ЈЁ4Ј©јУИлNa2CO3ИЬТєК±Ј¬И·ИПNi2+ТСѕНкИ«іБµнµДКµСй·Ѕ·ЁКЗ_______________ЎЈ
ЈЁ5Ј©ДіРЎЧйАыУГNiCO3ЦЖИЎДшЗвµзіШµДХэј«ІДБПјоКЅСх»ЇДш(NiOOH)Ј¬№эіМИзНјЈє

ўЩТСЦЄ 25ЎжК±Ј¬Ksp[Ni(OH)2]ЈЅ2ЎБ10-15Ј¬µ±µчЅЪ pHЎЭ9 К±Ј¬ИЬТєЦРІРБфµДc(Ni2+)________mol/LЎЈ
ўЪРґіцФЪїХЖшЦРјУИИNi(OH)2ЦЖИЎNiOOHµД»ЇС§·ЅіМКЅ________________ЎЈ
ўЫДшЗвµзіШµзЅвТєОЄ30%µДKOHЈ¬ёєј«ОЄMHЈЁјґОьЗвІДБПMОьёЅЗвФЧУЈ©ЎЈідµзК±ТІїЙКµПЦNi(OH)2ЧЄ»ЇОЄNiOOHЎЈЗлРґіц·ЕµзК±ёГµзіШµДЧЬ·ґУ¦КЅ______________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїПВБРёчЧйАлЧУФЪН¬Т»ИЬТєЦРТ»¶ЁДЬґуБї№ІґжµДКЗ(ЎЎЎЎ)
A. є¬ґуБїBa2Ј«µДИЬТєЦРЈєClЈЎўKЈ«ЎўSO![]() ЎўCO
ЎўCO![]()
B. є¬ґуБїHЈ«µДИЬТєЦРЈєMg2Ј«ЎўNaЈ«ЎўCO![]() ЎўSO
ЎўSO![]()
C. є¬ґуБїOHЈµДИЬТєЦРЈєKЈ«ЎўNOЎўSO![]() ЎўCu2Ј«
ЎўCu2Ј«
D. є¬ґуБїNaЈ«µДИЬТєЦРЈєHЈ«ЎўKЈ«ЎўSO![]() ЎўNO
ЎўNO
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїµзЅв·Ёґ¦АнCO2єНSO2»мєПОЫИѕЖшµДФАнИзПВНјЛщКѕЈ¬µзЅвЦКОЄИЫИЪМјЛбСОєНБтЛбСОЈ¬НЁµзТ»¶ОК±јдєуЈ¬Niµзј«±нГжРОіЙІфФУБтµДМј»эІгЎЈПВБРЛµ·ЁґнОуµДКЗ

A. Niµзј«±нГж·ўЙъБЛ»№Ф·ґУ¦
B. Сфј«µДµзј«·ґУ¦ОЄЈє2O2ЎЄ-4e-=O2
C. µзЅвЦКЦР·ўЙъµДАлЧУ·ґУ¦УРЈє2SO2 +4O2ЎЄ=2SO42ЎЄ
D. ёГ№эіМКµПЦБЛµзЅвЦКЦРМјЛбСОєНБтЛбСОµДЧФІ№ідС»·
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎї·дЅєКЗТ»ЦЦМмИ»ї№°©Т©Ј¬ЦчТЄ»оРФіЙ·ЦОЄї§·ИЛб±ЅТТхҐ(J)ЎЈєПіЙ»ЇєПОпIµДВ·ПЯИзПВЈє

ТСЦЄЈєўЩ
ўЪRCHO+HOOCCH2COOH![]() RCH=CHCOOH
RCH=CHCOOH
ўЫµ±фЗ»щУлЛ«јьМјФЧУПаБ¬К±Ј¬ТЧ·ўЙъЧЄ»ЇЈєRCH-CHOH=RCHCHO
Зл»ШґрПВБРОКМвЈє
(l)»ЇєПОпFµДГыіЖКЗ____;B-CµД·ґУ¦АаРНКЗ____ЎЈ
(2)»ЇєПОпEЦРє¬Сх№ЩДЬНЕµДГыіЖКЗ____Ј»G-HµД·ґУ¦ЛщРиКФјБєНМхјю·Ц±рКЗ____Ўў____ЎЈ
(3)Рґіц»ЇєПОпCУлРВЦЖCu(OH)2РьЧЗТє·ґУ¦µД»ЇС§·ЅіМКЅ____ЎЈ
(4)»ЇєПОпWУлE»ҐОЄН¬·ЦТм№№МеЈ¬БЅХЯЛщє¬№ЩДЬНЕЦЦАаєНКэДїНкИ«ПаН¬Ј¬ЗТ±Ѕ»·ЙПУР3ёцИЎґъ»щЈ¬ФтWїЙДЬµДЅб№№УР____ЦЦ(І»їјВЗЛі·ґТм№№)Ј¬ЖдЦРєЛґЕ№ІХсЗвЖЧПФКѕУР6ЦЦІ»Н¬»ЇС§»·ѕіµДЗвЈ¬·еГж»э±ИОЄ2:2:1Јє1:1:1Ј¬Рґіц·ыєПТЄЗуµДWµДЅб№№јтКЅ____ЎЈ
(5)ІОХХЙПКцєПіЙВ·ПЯЈ¬ЙијЖУЙCH3CH=CH2єНHOOCCH2COOHОЄФБПЦЖ±ё CH3CH2CH=CHCOOHµДєПіЙВ·ПЯ(ОЮ»ъКФјБИОСЎ)____ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎї·ґУ¦3AЈЁsЈ©+3BЈЁgЈ©=2CЈЁgЈ©+DЈЁgЈ©Ј¬ѕ3 minЈ¬BµДЕЁ¶ИјхЙЩ0Ј®9 molЎ¤L-1ЎЈ¶ФґЛ·ґУ¦ЛЩВКµД±нКѕХэИ·µДКЗЈЁ Ј©
AЈ®УГA±нКѕµД·ґУ¦ЛЩВККЗ0Ј®4 molЎ¤L-1Ў¤min-1
BЈ®·Ц±рУГBЎўCЎўD±нКѕµД·ґУ¦ЛЩВКЦ®±ИКЗ3ЎГ2ЎГ1
CЈ®ФЪ2 minД©µД·ґУ¦ЛЩВКЈ¬УГB±нКѕКЗ0Ј®3 molЎ¤L-1Ў¤min-1
DЈ®ФЪ2 minДЪµД·ґУ¦ЛЩВКЈ¬УГC±нКѕКЗ0Ј®3 molЎ¤L-1Ў¤min-1
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїDКЗТ»ЦЦґЯГЯТ©Ј¬FКЗТ»ЦЦПгБПЈ¬ЛьГЗµДєПіЙВ·ПЯИзПВЈє
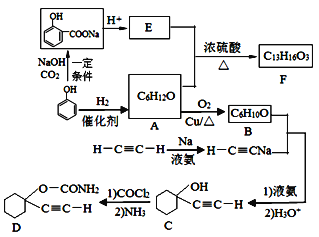
(1)AµД»ЇС§ГыіЖКЗ__________Ј¬CЦРє¬Сх№ЩДЬНЕµДГыіЖОЄ__________ЎЈ
(2)FµДЅб№№јтКЅОЄ______Ј¬AєНEЙъіЙFµД·ґУ¦АаРНОЄ______ЎЈ
(3)AЙъіЙBµД»ЇС§·ЅіМКЅОЄ_______________ЎЈ
(4) BУлТТИІДЖєПіЙCµД·ґУ¦АаРН(Лб»ЇЗ°)КЗ_________Ј»РґіцУЙCєПіЙDµДµЪ¶юёц·ґУ¦µД»ЇС§·ЅіМКЅ_____________ЎЈ
(5)Н¬К±ВъЧгПВБРМхјюµДEµДН¬·ЦТм№№МеУР__________ЦЦ(І»є¬БўМеТм№№)ЎЈ
ўЩУцFeCl3ИЬТє·ўЙъПФЙ«·ґУ¦Ј» ўЪДЬ·ўЙъТшѕµ·ґУ¦
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎї»ШґрПВБРОКМвЈє
ЈЁ1Ј©Ѕ«µИМе»эµИОпЦКµДБїЕЁ¶ИµДґЧЛбєНЗвСх»ЇДЖИЬТє»мєПєуЈ¬ИЬТєіК_______ЈЁМоЎ°ЛбРФЎ±Ј¬Ў°ЦРРФЎ±»тЎ°јоРФЎ±Ј¬ПВН¬Ј©Ј¬ИЬТєЦРc(NaЈ«) ____________c(CH3COOЈ)ЈЁМоЎ° >Ў±»тЎ°ЈЅЎ±»тЎ°<Ў± Ј©ЎЈ
ЈЁ2Ј©іЈОВПВЈ¬ИЎ0.2 mol/L HClИЬТєУл0.2mol/L MOHИЬТєµИМе»э»мєПЈ¬ІвµГ»мєПєуИЬТєµДpH=5ЎЈРґіцMOHµДµзАл·ЅіМКЅЈє__________________ЎЈ
ЈЁ3Ј© 99 ЎжК±Ј¬ПтpHЈЅ6µДХфБуЛ®ЦРјУИлNaHSO4ѕ§МеЈ¬±ЈіЦОВ¶ИІ»±дЈ¬ІвµГИЬТєµДpHЈЅ2ЎЈґЛК±Л®µДАлЧУ»эKwЈЅ________Ј¬Л®µзАліцµДcЈЁHЈ«Ј©ЈЅ________Ј¬¶шґЛК±ИЬТєЦРµДcЈЁNaЈ«Ј©__________ cЈЁSO42-Ј©ЈЁМоЎ°>Ў±Ў°ЈЅЎ±»тЎ°<Ў±Ј©ЎЈ
ЈЁ4Ј©ПаН¬ОВ¶ИПВµИОпЦКµДБїЕЁ¶ИµДПВБРИЬТєЦР
AЈ®NH4C1 BЈ®NH4HCO3 CЈ®NH4HSO4 DЈ®(NH4)2SO4
ўЩpHЦµУЙґуµЅРЎµДЛіРтКЗ__________(УГ¶ФУ¦µДЧЦДёМоРґЈ©ЎЈ
ўЪNH4+АлЧУЕЁ¶ИУЙґуµЅРЎµДЛіРтКЗ__________(УГ¶ФУ¦µДЧЦДёМоРґЈ©ЎЈ
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com