
(10��)ij��ѧ�С�������ͼ��ʾװ�ã����ּг�װ������ȥ��ʵ�飬��̽����ʪ��Cl2��Na2CO3��Ӧ�IJ��
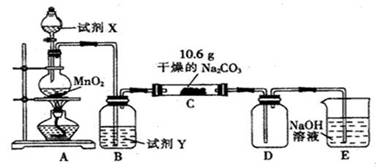
(1)д��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��________ _____________��
(2)д���Լ�Y�����ƣ�__________________ _________________��
(3)��C�з�Ӧ��Ĺ������ʷֳ����ȷݷ�װ����֧�Թ��У�
��������һ֧�Թ��м�����ˮ��������ȫ�ܽ�μ�BaCl2��Һ�������������ٵμ�NaOH��Һ����ǣ�д����������������ӷ���ʽ��________ ____________���ɴ������ƶϹ�������к���______ ____���ѧʽ����ͬ����
������һ֧�Թ��еμӹ�����ϡ���ᣬ����ɫ��ζ�������������Һ���壬������Һ�μӹ�����AgNO3��Һ����Һ����ǣ������ˡ�ϴ�ӡ�����õ�14.35g���壬�ɴ������ƶϹ�������к���____ ____
(4)��֪C����0.1molCl2�μӷ�Ӧ��D���ռ������������ȵ�һ���������(4)���������ݿ���֪��C�з�Ӧ�Ļ�ѧ����ʽΪ________ ___________��
��1�� MnO2+4H++2Cl-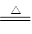 Mn2++Cl2��+2H2O(2��)
Mn2++Cl2��+2H2O(2��)
(2)����ʳ��ˮ�����Ȼ�����Һ��(2��)
(3)��HCO3����Ba2����OH����BaCO3����H2O(��2��); NaHCO3 ��NaCl (��1��)
(4)2Cl2+2Na2CO3+H2O=2NaHCO3+2NaCl ��Cl2O��(2��)
����������1��Aװ������ȡ�����ģ����Է���ʽ�� MnO2+4H++2Cl-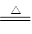 Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
��2���������ɵ������к����Ȼ��⣬���Ȼ�������̼���Ʒ�Ӧ������B���Լ��dz�ȥ�����е��Ȼ���ģ�ѡ����Լ��DZ���ʳ��ˮ��
��3���ټ����Ȼ���û�а�ɫ�������ɣ����Բ�����CO32�������μ�NaOH��Һ����ǣ����Ժ���HCO3�����������̼�����ƣ���Ӧ�ķ���ʽ��HCO3����Ba2����OH����BaCO3����H2O��
�ڼ��������ữ���������ܲ�����ɫ��������˳������Ȼ���������һ�������Ȼ��ơ�
��4����ɫ�����Ȼ�����0.1mol���������ɵ��Ȼ���Ҳ��0.1mol���μӷ�Ӧ��������0.1mol�����Ը��ݵ��ӵ�ʧ�غ��֪��������������Ԫ�صĻ��ϼ���+1�ۣ����Ը�������Ļ�ѧʽ��Cl2O����˷�Ӧ�ķ���ʽ��2Cl2+2Na2CO3+H2O=2NaHCO3+2NaCl ��Cl2O����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
| ||
| ||
| 3 |
| 4 |
| 3 |
| 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
| ||
| ||
| 3 |
| 4 |
| 3 |
| 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ����һ�з�У����10��ѧϰ������ϻ�ѧ�Ծ����������� ���ͣ�ʵ����
(10��)ij��ѧ�С�������ͼ��ʾװ�ã����ּг�װ������ȥ��ʵ�飬��̽����ʪ��Cl2��Na2CO3��Ӧ�IJ��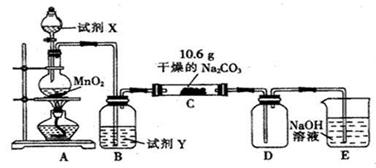
(1)д��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��________ _____________��
(2)д���Լ�Y�����ƣ�__________________ _________________��
(3)��C�з�Ӧ��Ĺ������ʷֳ����ȷݷ�װ����֧�Թ��У�
��������һ֧�Թ��м�����ˮ��������ȫ�ܽ�μ�BaCl2��Һ�������������ٵμ�NaOH��Һ����ǣ�д����������������ӷ���ʽ��________ ____________���ɴ������ƶϹ�������к���______ ____���ѧʽ����ͬ����
������һ֧�Թ��еμӹ�����ϡ���ᣬ����ɫ��ζ�������������Һ���壬������Һ�μӹ�����AgNO3��Һ����Һ����ǣ������ˡ�ϴ�ӡ�����õ�14.35g���壬�ɴ������ƶϹ�������к���____ ____
(4)��֪C����0.1molCl2�μӷ�Ӧ��D���ռ������������ȵ�һ���������(4)���������ݿ���֪��C�з�Ӧ�Ļ�ѧ����ʽΪ________ ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�걱���и߿���ѧģ���Ծ��������������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com