��������ƣ�Na
2S
2O
3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã�Na
2SO
3+S�TNa
2S
2O
3����������Һ����������ΪNa
2S
2O
3?5H
2O��Na
2S
2O
3?5H
2O��40��45���ۻ���48��ֽ⣻Na
2S
2O
3������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ��������ͼ1��ʾ��
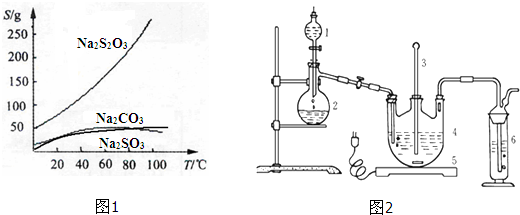
���ְ����·����Ʊ�Na
2S
2O
3?5H
2O��
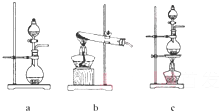
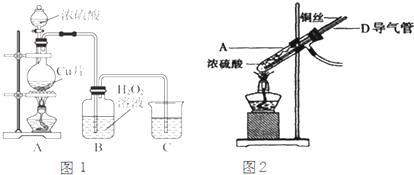
�����ƺ�̼���ư���ӦҪ�����һ������������ƿ�У�ע��150mL����ˮʹ���ܽ⣬�ڷ�Һ©���У�ע��Ũ���ᣬ��װ��2�м����������ƹ��壬����ͼ2��װ��װ�ã�
��1������2������Ϊ
��װ��6�пɷ���
��
A��BaCl
2��Һ B��ŨH
2SO
4 C������KMnO
4��Һ D��NaOH��Һ
��2����Һ©��������ע��Ũ����ʹ��Ӧ�����Ķ�����������Ͼ��ȵ�ͨ��Na
2S��Na
2CO
3�Ļ����Һ�У����ô������������������ȣ���Ӧԭ��Ϊ��
��Na
2CO
3+SO
2�TNa
2SO
3+CO
2 ��Na
2S+SO
2+H
2O�TNa
2SO
3+H
2S
��2H
2S+SO
2�T3S��+2H
2O ��Na
2SO
3+S
Na
2S
2O
3����SO
2�����ͨ�룬������Һ���д���dz��ɫ��������������ͨSO
2���壬��ӦԼ��Сʱ������Һ��PH�ӽ���С��7ʱ������ֹͣͨ���ͼ��ȣ���ҺPHҪ���Ʋ�С��7�������ǣ�
�������ֺ�������ӷ���ʽ��ʾ����
����Na
2S
2O
3?5H
2O���ⶨ������

��3��Ϊ���ٲ�Ʒ����ʧ��������Ϊ
���������dz���ϴ�Ӹ������ϴ�Ӳ�������
�����Լ�����ϴ�Ӽ���
��4������Ũ����Һֱ����Һ����ɫ����Ϊֹ������ʱΪʲôҪ�����¶Ȳ��˹���
��
��5���ƵõĴ־��������������������ʣ�Ϊ�˲ⶨ�ֲ�Ʒ��Na
2S
2O
3?5H
2O�ĺ�����һ�������������������KMnO
4��Һ�ζ��ķ������ٶ��ֲ�Ʒ������������KMnO
4��Һ����Ӧ������ȡ1.28g�Ĵ���Ʒ����ˮ����0.40mol/L KMnO
4��Һ���������������ữ���ζ�������Һ��S
2O
32-ȫ��������ʱ������KMnO
4��Һ���20.00mL����5S
2O
32-+8MnO
4-+14H
+�T8Mn
2++10SO
42-+7H
2O���Իش�
��KMnO
4��Һ����
�����ʽ����ʽ�����ζ����У�
�ڵζ��յ�ʱ����ɫ�仯��
��
�۲�Ʒ��Na
2S
2O
3?5H
2O����������Ϊ
��



 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�


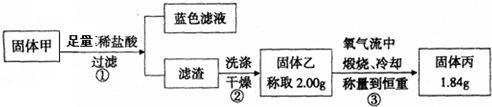
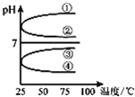 A��B��C��D��E������Һ�ֱ���NaOH��NH3?H2O��CH3COOH��HCl��NH4HSO4�е�һ�֣������½�������ʵ�飺
A��B��C��D��E������Һ�ֱ���NaOH��NH3?H2O��CH3COOH��HCl��NH4HSO4�е�һ�֣������½�������ʵ�飺