
| ||

| 5 |
| 8 |
| 248g/mol��0.005mol |
| 1.28g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���е㣺X2Y��X2W |
| B����X��Y��Z��W����Ԫ����ɵĻ�����Ⱥ��й��ۼ��ֺ����Ӽ� |
| C��ԭ�Ӱ뾶��X��Y��Z��W��R |
| D��Y��W�γɵĻ�����WY2���γ��������Ҫ����֮һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
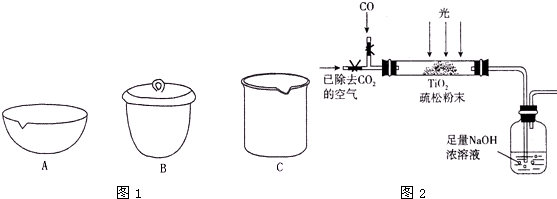
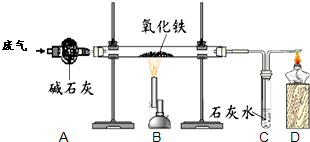 ij����С���ͬѧ�ռ��˺�ˮ������һ����̼�Ͷ�����̼�ķ�����Ϊȷ�����ַ����д���CO��������ʵ���Ұ���ͼ��ʾװ�ý���ʵ�顲����ͨ��װ��A�ٶȺ����������ڴ˴������ķ�Ӧ��ȫ����ʯ�ң�CaO��NaOH�Ļ�����������
ij����С���ͬѧ�ռ��˺�ˮ������һ����̼�Ͷ�����̼�ķ�����Ϊȷ�����ַ����д���CO��������ʵ���Ұ���ͼ��ʾװ�ý���ʵ�顲����ͨ��װ��A�ٶȺ����������ڴ˴������ķ�Ӧ��ȫ����ʯ�ң�CaO��NaOH�Ļ������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
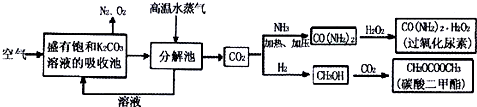
| ���ۼ� | C=O | H-H | C-O | C-H | O-H |
| ����KJ?mol-1 | 750 | 436 | 358 | 413 | 463 |
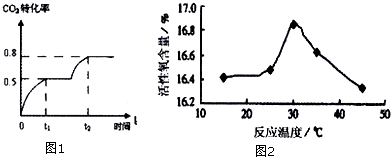
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ں��д���I-���ӵ���Һ�У�Cl-��Fe3+��Na+��Mg2+ |
| B������ˮ�������c��H+��=10-12mol?L-1 ����Һ�У�Na+��Ba2+��Cl-��Br- |
| C��ʹ���ȳʺ�ɫ����Һ�У�Fe2+��Na+��SO42-��ClO- |
| D���ڼ���Al�ܷų�����H2����Һ�У�NH4+��SO42-��Cl-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���Ͽ�Ƭ | ||
| ���� | �۵� | �е� |
| SiCl4 | -70�� | 57.6�� |
| TiCl4 | -25�� | 136�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com