
| A������Һ����ˮ�����c��H + �� һ��С�� 10 -7 mol/L |
| B������Һ��0.1 mol?L-1��CH3COOH��Һ������ʵ���Ũ�ȡ��������NaOH��Һ��϶��� |
| C������Һ�е����ʿ�����CH3COOH��CH3COONa |
| D����������Һ�м����������ᣬ��ʹ��Һ������Ũ�ȸı�Ϊc��CH3COO-����c��H+����c��Na+����c��OH-�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ϵ��������ܶȷ����仯��˵����Ӧû�дﵽƽ��״̬ |
| B����ϵ�и���ֵ�������������仯��˵��ƽ�ⷢ�����ƶ� |
| C����S��0�ķ�Ӧһ�����Է���Ӧ |
| D����Ӧ�ﵽƽ��״̬ʱ�������淴Ӧ���ʾ�Ϊ0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����Թ�ȡ��Na2CO3��Һ������ȡ�����࣬Ϊ�˲��˷ѣ��ְѹ������Լ������Լ�ƿ�� |
| B��Ba��NO3��2 ����ˮ���ɽ�����Ba��NO3��2 �ķ�Һ����ˮ���У�����ˮ������ˮ�� |
| C������������ʹNaCl ����Һ������ʱ��Ӧ����������NaCl ��Һȫ���������� |
| D����ҩ����ֽ�۰ѷ�ĩ״ҩƷ�����Թܵĵײ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
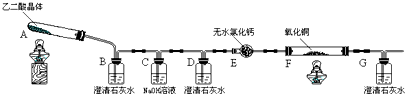
| �ζ�ǰ | ��һ���յ� | �ڶ����յ� | �������յ� | |
| �ζ���Һ��̶� | 0.00mL | 16.02mL | 16.00mL | 16.01mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | �ܶ�/��g?mL-1�� | �е� | ˮ���� | �ܽ��� |
| �� | 0.893 | 78.5�� | �� | ������ |
| �� | 1.220 | 100.7�� | �� | ���ڼ� |
| A������ | B������ | C����Һ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
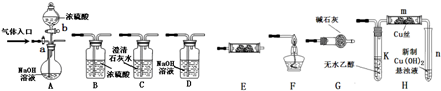
 �ⶨһ����������п�Ͻ���ǿ����Һ��Ӧ�����������������������úϽ�������п��������������������ʵ����Ʒ��800mL�ձ���100mL��Ͳ���̾�©����ͭ������п�Ͻ���Ʒ��Ũ���ᣨ�ܶ�1.19g/L����ˮ����ͼʾװ�ý���ʵ�飬�ش��������⣮����Ͻ���Ʒ��ȫ��Ӧ���������������������100mL��
�ⶨһ����������п�Ͻ���ǿ����Һ��Ӧ�����������������������úϽ�������п��������������������ʵ����Ʒ��800mL�ձ���100mL��Ͳ���̾�©����ͭ������п�Ͻ���Ʒ��Ũ���ᣨ�ܶ�1.19g/L����ˮ����ͼʾװ�ý���ʵ�飬�ش��������⣮����Ͻ���Ʒ��ȫ��Ӧ���������������������100mL���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��20% | B��30% |
| C��70% | D��80% |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com