
����Ŀ����Դ��������������ͷ�չ����Ҫ���ʻ�����������Դ�ĺ������ú�����Դ�ĺ��������ǵ���������ٵ��Ͼ����⣬�ش��������⣺
��1���Ҵ���C2H5OH����δ����ȼ������ѡ������Һ��ȼ�ϡ�1 g�Ҵ���ȫȼ������Һ̬ˮ�ų�a kJ�����������Ҵ�ȼ�յ��Ȼ�ѧ����ʽΪ__________��
��2������C3H8(g)= C3H6(g)+H2(g) H=+bkJmol��b��0���ķ�Ӧ�У���Ӧ����е�������________������������������������С��������������е�����������ô�ڻ�ѧ��Ӧʱ����Ӧ�����Ҫ________�������ų�����������������������ת��Ϊ�����
��3��������ˮ��ȡ������Դ�����������о�������ȷ����________��
A ���ˮ����������ǿ���ȼ�յ����ʣ���˿��о���ˮ���ֽ������£�ʹ���Ϊ������Դ
B �跨��̫����۽����������£�ʹˮ�ֽ��������
C Ѱ�Ҹ�Ч������ʹˮ�ֽ����������ͬʱ�ͷ�����
D Ѱ��������������ڿ���������Դ���Էֽ�ˮ��ȡ����
��4����֪���������Ȼ�ѧ����ʽ��
A 2H2(g)+O2(g)=2H2O(l) H = -571.6kJmol-1
B C3H8(g)+5O2(g)=3CO2(g)+4H2O(l) H= -2220kJmol-1
�ܱ�ʾȼ���ȵ��Ȼ�ѧ����ʽΪ________����A��B�����������22.4L��C3H8��H2������壨����H2���������Ϊ1/2������������������ȫȼ�գ���ų�������Ϊ________kJ��
���𰸡�C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ��H=46a kJmol1 С�� ���� AC B 1252.9kJ
��������
��1����֪1 g�Ҵ���ȫȼ������Һ̬ˮ�ų�a kJ����������1mol�Ҵ�ȼ�շų�46a kJ��������
��2����Ӧ��������С����������������Ϊ���ȷ�Ӧ��
��3��A. ������ȼ�գ���ˮ�ֽ�����������
B. ̫����۽����������£������Ļ�ʯ��Դ����ʹˮ�ֽ����������
C. ˮ�ֽ���Ҫ����������
D. Ѱ��������������ͳɱ���
��4��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ�������ʱ���ų���������һ��C��CO2(g)��S��SO2(g)��H��H2O(l)���ȼ�������22.4L��C3H8��H2���������C3H8�����ʵ�����H2�����ʵ�����Ϊ0.5mol���ٽ���Ȼ�ѧ����ʽ����ų���������
��1����֪1 g�Ҵ���ȫȼ������Һ̬ˮ�ų�a kJ����������1mol�Ҵ�ȼ�շų�46a kJ�����������Ҵ�ȼ�յ��Ȼ�ѧ����ʽΪC2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ��H=46a kJmol1��
�ʴ�Ϊ��C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ��H=46a kJmol1��
��2����֪��H=+bkJmol1(b>0)���÷�ӦΪ���ȷ�Ӧ����Ӧ����е�������С����������е�����������ô�ڻ�ѧ��Ӧʱ����Ӧ�����Ҫ������������ת��Ϊ�����
�ʴ�Ϊ��С�ڣ����գ�
��3��A. ������ȼ�գ���ˮ�ֽ��������������ֽⲻ�ܵõ���������A����
B. ̫����۽����������£������Ļ�ʯ��Դ����ʹˮ�ֽ������������B��ȷ��
C. ˮ�ֽ���Ҫ���������������ͷ���������C����
D. Ѱ��������������ͳɱ������ڿ���������Դ���Էֽ�ˮ��ȡ��������D��ȷ��
�ʴ�Ϊ��AC��
��4��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ�������ʱ���ų���������һ��C��CO2(g)��S��SO2(g)��H��H2O(l)��A������Ϊ2mol��B��C3H8Ϊ1mol�������ɶ�����̼�����Һ̬ˮ�����ܱ�ʾȼ���ȵ��Ȼ�ѧ����ʽ��ΪB�������22.4L��C3H8��H2������壬��1mol������H2���������Ϊ1/2����C3H8�����ʵ�����H2�����ʵ�����Ϊ0.5mol������������������ȫȼ�գ��ų�������Ϊ![]() ��
��
�ʴ�Ϊ��B��1252.9kJ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڷ�Ӧ�ȵ���������ȷ���ǣ� ��
A.HCl��NaOH��Ӧ���к�����H��-57.3kJ/mol����H2SO4��Ba(OH)2��Ӧ����1molH2Oʱ��ų�57.3kJ������
B.�����ȼ����Ϊ890.3kJ��mol-1�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪCH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H��-890.3kJ��mol-1
C.һ�������£���0.5molN2��l.5molH2�����ܱ������г�ַ�Ӧ����NH3������19.3kJ�������Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)![]() 2NH3(g) ��H��-38.6kJ��mol-1
2NH3(g) ��H��-38.6kJ��mol-1
D.��S(s)+O2(g)��SO2(g) ��H1��S(g)+O2(g)��SO2(g) ��H2����H1<��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£������ΪV L���ܱ������з�����ѧ��ӦCO(g)��2H2(g) ![]() CH3OH(g)�����жϸÿ��淴Ӧ�ﵽ��ѧƽ��״̬�ı�־��(����)
CH3OH(g)�����жϸÿ��淴Ӧ�ﵽ��ѧƽ��״̬�ı�־��(����)
A.v����(CH3OH)��v����(CO)B.���������ܶȲ��ٸı�
C.��������ƽ����Է����������ٸı�D.CO��H2��CH3OH��Ũ�ȱ�Ϊ1:2:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��ȥ�����е�Ca2+��Mg2+��Fe3+��SO42+�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
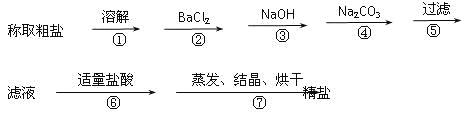
(1)�ж�BaCl2�ѹ����ķ�����_________________________��
(2)�������У���ص����ӷ���ʽ��___________��__________��
(3)�����������pHֵ�ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����_____________��
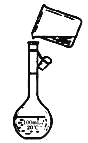
(4)Ϊ���龫�δ��ȣ�������150 mL.0.2 mol/L NaCl(����)��Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£��������ؾ����������ṹ�����������Ļ��ϼ۲���Ϊ0�ۣ�����Ϊ��2�ۡ���ͼ��ʾΪ�������ؾ����һ��������������˵����ȷ����

A���������صĻ�ѧʽΪKO2��ÿ����������4��K����4��O2-
B��������ÿ��K����Χ��8��O2-��ÿ��O2-��Χ��8��K��
C����������ÿ��K�����������K����8��
D����������ÿ��K�����������K����6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȥ���������е�����(������Ϊ����)�����Լ��Ͳ�����������ȷ�ģ� ��
��Cl2(HCl)������ʳ��ˮ������
��CO2(SO2)������Na2CO3��Һ��ϴ��
������(����)��NaOH��Һ������
��MnO2(KCl)��ˮ���ܽ�����
��̼��(�ⵥ��)��CCl4����Һ
��C2H5OH(H2O)��������CaO������
A.�٢ڢ�B.�ۢܢ�C.�ڢܢ�D.�٢ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ������У�һ�������£��������·�Ӧ��NO(g)��CO(g) ![]()
![]() N2(g)+CO2(g)����H= -373.2kJ/mol���ﵽƽ���Ϊ��߸÷�Ӧ�����ʺ�NO��ת���ʣ���ȡ����ȷ��ʩ��
N2(g)+CO2(g)����H= -373.2kJ/mol���ﵽƽ���Ϊ��߸÷�Ӧ�����ʺ�NO��ת���ʣ���ȡ����ȷ��ʩ��
A.�Ӵ���ͬʱ�����¶�B.�Ӵ���ͬʱ����ѹǿ
C.�����¶�ͬʱ����N2D.�����¶�ͬʱ����ѹǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��������������ԭ�����͵���
A.������ˮ��Һ�м����������S2-������
B.������������ڰ���������Ӧ
C.��ѹ�����ںϳɰ���Ӧ
D.��������ˮ�м���̼��������ڴ�����Ũ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������װ�ô�����KMnO4�ķ�Һ��ʹMnԪ��ת��ΪMnO2�������Ӷ������ؽ�����Ⱦ������˵���������
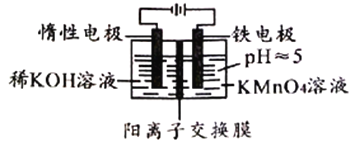
A. MnO4-������ȫ��ʵ�����ʱ�����ܻ����ɳ���
B. �Ҳ������Fe2+����MnO4-�����ӷ���ʽΪ��7H2O+3Fe2++MnO4-=3Fe(OH)3��+MnO2��+5H+
C. ����·��ת��6mole-ʱ�����Բ���87gMnO2����
D. Ϊ�������Ҳ���Һ�ĵ����Կ��Լ�ϡ�������ǿ���Ի���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com