
������������ȷ���ǣ� ��
��1��������̼�ظ������� ��2���ð�ˮ��ȥ�Թ��ϵ�����
��3�������������ά������̫���ܵ�ص���Ҫԭ��
��4������ϡ���ᡢ̼������Һ����������Һ���ʵ������֤Ԫ�صķǽ�����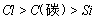
��5������ֻ�ܸı䷴Ӧ�Ļ�ܣ����ܸı䷴Ӧ����ЧӦ
��6����������̲������������������������跴Ӧ������
��7��ͬ����Ԫ�صļ������ӻ�ԭ��Խǿ��ˮ��̶�Խ��
��8��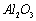 �ڹ�ҵ�������������²��ϣ�Ҳ���ڵ�ⷨ������
�ڹ�ҵ�������������²��ϣ�Ҳ���ڵ�ⷨ������
��9�������ЧӦ������������Һ�뽺�壬�ơ������ܲ��������ЧӦ
��10������������ˮ��������ɱ����̼�ᱵ�����ڱ�����
A����1����4����6����7�� B����4����6����9����10��
C����3����5����6����8�� D����5����6����8����9��
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л��ۺ�����������ȼ���������Ӿۺ����ʹ�ð�ȫ�ԣ�������Ӧ�÷�Χ�����磬��ij����ϩ��֬�м����������������Ʊ�����ȼ��Mg(OH)2����֬��ȼ�Դ�͡���Mg(OH)2�������������£�

�ž���±ˮ�е�MgCl2������ʯ���鷴Ӧ�ϳɼ�ʽ�Ȼ�þ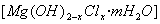 ����Ӧ�Ļ�ѧ����ʽΪ ��
����Ӧ�Ļ�ѧ����ʽΪ ��
�ƺϳɷ�Ӧ������393 K��523 K��ˮ�ȴ���8 h��������Ӧ��
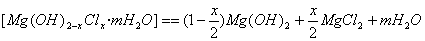
ˮ�ȴ������ˡ�ˮϴ��ˮϴ��Ŀ���� ��
����ȼ��Mg(OH)2���о�������ɢ����߷��Ӳ��������Ժõ��ص㡣������������������йصIJ����� ��
����֪�Ȼ�ѧ����ʽ��
Mg(OH)2(s)==MgO(s)+H2O(g)�� ��H1=+81.5 kJ��mol-1
Al(OH)3(s)=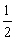 Al2O3(s)+
Al2O3(s)+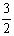 H2O(g)�� ��H2=+87.7 kJ��mol-1
H2O(g)�� ��H2=+87.7 kJ��mol-1
��Mg(OH)2��Al(OH)3����ȼ���õ���Ҫԭ���� ��
�ڵ�����Mg(OH)2��Al(OH)3��ȣ���ȼЧ���Ϻõ��� ��
ԭ���� ��
�ɳ�����ȼ����Ҫ�� ���ࣺA.±ϵ�����������飻B.��ϵ����������������C.���࣬��Ҫ��Mg(OH)2��Al(OH)3���ӻ����ĽǶȿ��ǣ�Ӧ��ʱ���������ȼ���� (�����)�������� ��
���ࣺA.±ϵ�����������飻B.��ϵ����������������C.���࣬��Ҫ��Mg(OH)2��Al(OH)3���ӻ����ĽǶȿ��ǣ�Ӧ��ʱ���������ȼ���� (�����)�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ� ��
A���ҹ������з��ġ���о1�š�CPUоƬ����ά��ͬ�ֲ���
B����Ҫͨ����ѧ��Ӧ���ܴӺ�ˮ�л��ʳ�κ͵�ˮ
C��ˮ�������Ͳ����ϵĴ��̶��ǹ�������Ʒ
D���ֹ��Ʊ��������漰������ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A+B��X+Y+ H2O(Ϊ��ƽ����Ӧ������ȥ)����ѧ������Ӧ�Ļ�ѧ����ʽ������A��B�����ʵ���֮��Ϊ1��4����ش�
��1����Y�ǻ���ɫ���壬��Y�ĵ���ʽ�� ���÷�Ӧ�Ļ�ѧ����ʽ��
��
��2����AΪ�ǽ������ʣ���������ԭ�Ӻ��������������Ǵ�����������2����B����ҺΪijŨ�ᣬ��Ӧ���������뻹ԭ�������ʵ���֮���� ��
��3����AΪ�������ʣ�������A��B��Ũ��Һ�С��ۻ�������A������X��Һ�У�
��AԪ�������ڱ��е�λ����______�����������ں��壩��Y�Ļ�ѧʽ��______��
�ں�a mol X����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X��______ mol��
��4����A��B��X��Y��Ϊ�����A����ˮ������������Ӻ�ˮ���õ�������ɾ���ˮ����A��Һ�м��������ữ��AgNO3��Һ��������ɫ������B����ɫΪ��ɫ������A����μ���B[i]�����ӷ���ʽ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£������и�����������Һ�У�һ���ܴ����������������
A����ʹpH��ֽ�ʺ�ɫ����Һ��Na����NH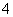 ��I����NO
��I����NO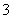
B��������������H2����Һ��K����Mg2����SO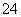 ��HCO
��HCO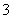
C��c(Fe3��)��0.1mol��L��1����Һ��H����Al3����I����SCN��
D��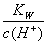 ��0.1mol��L��1����Һ��Na����K����SiO
��0.1mol��L��1����Һ��Na����K����SiO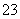 ��NO
��NO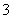
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪W��X��Y��ZΪ������Ԫ�أ�W��Zͬ���壬X��Y��Zͬ���ڣ�W����̬�⻯����ȶ��Դ���Z����̬�⻯����ȶ��ԣ�X��YΪ����Ԫ�أ�X�������ӵ�������С��Y�������ӵ������ԡ�����˵����ȷ���ǣ� ��
A��W����̬�⻯��ķе�һ������Z����̬�⻯��ķе�
B��W��X�γɵĻ�������ֻ�����Ӽ�
C��X��Y��Z��W��ԭ�Ӱ뾶���μ�С
D����W��Yԭ���������5��������γɻ�����Ļ�ѧʽһ��ΪY2W3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ķ�չ�봴������ֹ�����ҹ���ѧ�Һ�°�ĸ����Ĵ����������գ��������̿ɼ�Ҫ��ʾ��ͼK39��4��

ͼK39��4
(1)���������Ҫͨ��CO2�Ͱ�����Ӧѡͨ��______ (�ѧʽ)��ԭ����_________________________________________��
(2)�������з�����Ӧ�Ļ�ѧ����ʽ��__________________________________��
(3)ĸҺ�е�������Ҫ��________����ĸҺ��ͨ��������ϸСʳ�ο�������ȴ��������Ʒ��ͨ�백����������_____________________________________________��
(4)ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%���ϣ���Ҫ�������________(�����������еı��)��ѭ��������X��__________���ӳ���������ȡ�����IJ�����__________ ��
(5)д������¯�з�����Ӧ�Ļ�ѧ����ʽ_____________________________��
(6)�����ƵõIJ�Ʒ̼�����п��ܺ��е�������____________(�ѧʽ)��Ϊ������� �ʵĴ��ڣ����������__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��FeΪ������PtΪ�������������� Na2SO4 ��Һ���е�⣬һ��ʱ���õ�4 mol Fe(OH)3�������˼乲����ˮ�����ʵ���Ϊ ( )
A��6mol B��8mol C��10mol D��12mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������ȡCO2һ���Ϊ������裺�ټ��װ�õ������ԣ������ӡ���װ��������
��©����ע��ϡ���������ƿ�з������ʯ�����ռ����塣��ȷ�IJ���˳��
��( )
A���٢ڢۢܢ� B���٢ڢܢۢ�
C. �ڢ٢ۢܢ� D���ڢ٢ܢۢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com