
����Ŀ��A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȡ�
![]()
(1)д��A��B��C��Ԫ������________��________��________��
(2)C��Ԫ�����ڱ��е�λ����____________________��
(3)B��ԭ�ӽṹʾ��ͼΪ________________��C���⻯����B���⻯����ȶ���ǿ��˳��Ϊ________>________(�ѧʽ)��
(4)�Ƚ�A��C��ԭ�Ӱ뾶A________C��д��A����̬�⻯����A������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ______________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ�������Dz��������Լ����������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺
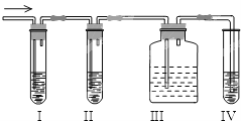
��1��װ��Ӧʢ�ŵ��Լ���I ____��IV _______���������й��Լ����������ո��ڣ���
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D������KMnO4��Һ
��2����˵��SO2������ڵ�������____________________________��
��3��ʹ��װ��II��Ŀ����____________________________________ ��
��4��ʹ��װ��III��Ŀ���� ___________________________________��
��5��ȷ��������ϩ��������___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڸ��������£�����ѡ����ʾ�����ʼ�ת������ʵ�ֵ���(����)
A. SiO2![]() SiCl4
SiCl4![]() Si B. CuSO4
Si B. CuSO4![]() Cu(OH)2
Cu(OH)2![]() Cu
Cu
C. ����NaCl(aq)![]() NaHCO3(s)
NaHCO3(s)![]() Na2CO3(s) D. FeS2(s)
Na2CO3(s) D. FeS2(s)![]() SO3(g)
SO3(g)![]() H2SO4
H2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����(����)
A. �ϳɰ���Ӧ�Ƿ��ȷ�Ӧ�����Թ�ҵ�ϲ��õ�����������߰��IJ���
B. ����ˮբ����������������ӵ����������������ӻ��丯ʴ
C. Na2O2��ˮ��Ӧ����1 mol O2��������ת�Ƶĵ�����ԼΪ4��6.02��1023
D. �����¶Ȳ��䣬��ϡ��ˮ�л���ͨ��CO2����Һ��![]() ��ֵ����
��ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����������Ϊԭ�������������ƣ��乤���������£�
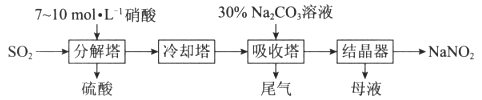
��֪��Na2CO3��NO��NO2===2NaNO2��CO2
(1)���ֽ�������SO2�������룬������������ܣ���Ŀ����_____________________________��
(2)���ֽ������е��¶Ȳ��˹��ߣ���ԭ����_____________________________________________��
(3)��һ���������ڡ��ֽ�������ͨSO2���������ᣬ����Ӧ�����ɵ�NO��NO2���ʵ���֮��ǡ��1��1���ֽ������з�����Ӧ�Ļ�ѧ����ʽΪ______________________________________________��
(4)���ֽ������е�����Ũ�Ȳ��˹����ԭ����___________________________________________��
(5)Ϊ��ߵ��������ת���ʣ�����β������ͨ��һ������________���壬��ͨ�롰���������У�ʵ��ѭ�����ա�
(6)����������������Һ�г�����NaNO2��NaNO3������Na2CO3�⣬�����е�����Ϊ________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������þ��Ϊԭ����ȡMgSO4��7H2O�ֲ�Ʒ�Ĺ������£�
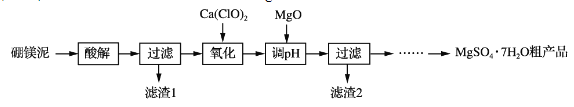
��þ�����Ҫ�ɷ����±���
MgO | SiO2 | FeO��Fe2O3 | CaO | Al2O3 | B2O3 |
30%��40% | 20%��25% | 5%��15% | 2%��3% | 1%��2% | 1%��2% |
(1)����⡱ʱΪ���Mg2���Ľ����ʣ��ɲ��õĴ�ʩ��_____(дһ��)��������˹���̫���ԭ����_____��
(2)�������������У�����H2O2����Ca(ClO)2��������Ӧ�����ӷ���ʽΪ______________��ʵ��δʹ��H2O2����H2O2�ɱ����⣬�����ܵ�ԭ����______________________________________��
(3)����pH��ʱ��MgO������NaOH��Һ��ԭ����________________________��
(4)��ϸ�����Ϣ����MgSO4��7H2O�ֲ�Ʒ(������CaSO4)�ᴿ��ȡMgSO4��7H2O��ʵ�鷽�����£����ֲ�Ʒ����ˮ��_________________________________________________����������Ȼ�ӷ����(ʵ���б���ʹ�õ��Լ��У�����MgSO4��Һ���Ҵ�)���������ε��ܽ��(g/100 gˮ)
�¶ȡ� | 10 | 30 | 40 | 50 |
CaSO4 | 0.19 | 0.21 | 0.21 | 0.20 |
MgSO4��7H2O | 30.9 | 35.5 | 40.8 | 45.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��G(�����ᱡ�ɴ���)��һ���������ಡ��ҩ���ϳ���·���£�
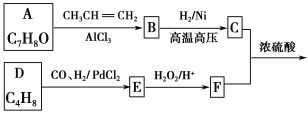

��֪����A�����Ȼ����ٶ��Է�����ɫ��Ӧ
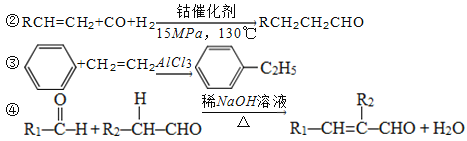
��1��A������Ϊ________��
��2��G�к�������������Ϊ________��
��3��D�ķ����к���________�ֲ�ͬ��ѧ��������ԭ�ӡ�
��4��E�����Ƶ�������ͭ��Ӧ�Ļ�ѧ����ʽΪ��______________________________��
��5��д����������������A��ͬ���칹��Ľṹ��ʽ����дһ�֣���___________��
a����������6��̼ԭ����һ��ֱ���ϣ�
b���������OH��
��6������ȩ���������ϡ��ٽ����ȣ�д�����Ҵ�Ϊԭ���Ʊ�CH3(CH2)3CHO�ĺϳ�·������ͼ(���Լ���ѡ)��___________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�絼��Խ������Խǿ����������0.100 mol��L-1NaOH��Һ�ֱ�ζ�10.00mLŨ�Ⱦ�Ϊ0.100 mol��L-1������ʹ�����Һ����õζ���������Һ�ĵ絼����ͼ��ʾ������˵����ȷ����
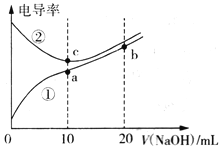
A. ���ߢٴ����ζ����������
B. a����Һ��: c(OH-)+c(CH3COO-)-c(H+) =0.1 mol/L
C. a��b��c������Һ��ˮ�ĵ���̶ȣ�c>a>b
D. b����Һ�У�c(OH-)>c(H+)+c(CH3COO-)+c(CH3COOH)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڴ��Թ������3mL�Ҵ���2mL�����ᣬ�ٻ�������2mLŨ���ᣬ�ӱ�������һ֧�Թ��м��뱥��̼������Һ���Իش��������⣺
��1��������Ӧ�����壩��__����Ӧ����__��Ӧ��ѧ����ʽ��__��
��2����������Ӧ�У�����ķ��ӽṹ����ʲô�仯_________��
��3��������Ӧ�ڳ����·�Ӧ������һ��15����ܴﵽƽ�⣬������ʹ��Ӧ�ӿ���_________��
��4��������Ӧ�У�Ũ������ʲô����_________��
��5��Ϊʲô�������շ�Ӧ��������Թ���Ҫװ����̼������Һ_________�����ñ���̼������Һ������ˮ������������Ӧ�����������ʲô��ͬ�Ľ��_________��
��6��Ϊʲô�������ܿڲ��ܲ���̼����Һ����_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com