
ЁОЬтФПЁПБНАЭБШЭзЪЧеђОВЁЂАВУпРрвЉЮя,ЦфКЯГЩТЗЯпШчЯТ:
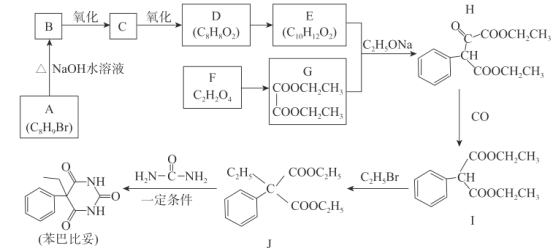
ЧыЛиД№ЯТСаЮЪЬт:
(1)GЕФЛЏбЇУћГЦЮЊ_________,CЕФЗжзгЪНЮЊ__________ЁЃ
(2)F+XЁњG+ H2O,XЕФНсЙЙМђЪНЮЊ____________ЁЃ
(3)HжаКЌгаЕФЙйФмЭХУћГЦЮЊ__________,IЁњJЕФЗДгІРраЭЮЊ______________ЁЃ
(4)ЭЌЪБТњзуЯТСаЬѕМўЕФEЕФЭЌЗжвьЙЙЬхЙВга______жжЁЃ
ЂйКЌгаБНЛЗЧвБНЛЗЩЯгаСНИіШЁДњЛљ
ЂкФмЗЂЩњЫЎНтЗДгІЧвФмЗЂЩњвјОЕЗДгІ
(5)ЛЏКЯЮяJЁњБНАЭБШЭзЕФЛЏбЇЗНГЬЪНЮЊ________________________________ЁЃ
(6)вд1,3-ЖЁЖўЯЉЁЂввДМКЭФђЫи(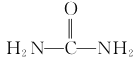 )ЮЊдСЯКЯГЩ
)ЮЊдСЯКЯГЩ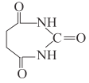 ЫљашЮоЛњЪдМСШЮбЁ,ЧыаДГіСїГЬ:_____________________________
ЫљашЮоЛњЪдМСШЮбЁ,ЧыаДГіСїГЬ:_____________________________
ЁОД№АИЁПввЖўЫсЖўввѕЅ C8H8O CH3CH2OH ѕЅЛљЁЂєЪЛљ ШЁДњЗДгІ 15 ![]() +
+ +2C2H5OH CH2=CHЃCH=CH2
+2C2H5OH CH2=CHЃCH=CH2![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br ![]() HOCH2CH2CH2CH2OH
HOCH2CH2CH2CH2OH![]() HOOCCH2CH2COOH
HOOCCH2CH2COOH![]() C2H5OOCCH2CH2COOC2H5
C2H5OOCCH2CH2COOC2H5 
ЁОНтЮіЁП
AЗжзгЪНЮЊC8H9BrЃЌИљОнHНсЙЙМђЪНЃЌAжагІКЌгаБНЛЗЃЌAдкЧтбѕЛЏФЦЫЎШмвКЩњГЩBЃЌЗЂЩњТБДњЬўЕФЫЎНтЃЌєЧЛљШЁДњЃBrЕФЮЛжУЃЌBбѕЛЏГЩCбѕЛЏГЩDЃЌетЪЧДМБЛбѕЛЏГЩШЉЃЌШЉБЛбѕЛЏГЩЫсЃЌМДAЕФНсЙЙМђЪНЮЊ![]() ЃЌBЕФНсЙЙМђЪНЮЊ
ЃЌBЕФНсЙЙМђЪНЮЊ![]() ЃЌCЕФНсЙЙМђЪНЮЊ
ЃЌCЕФНсЙЙМђЪНЮЊ![]() ЃЌDЕФНсЙЙМђЪНЮЊ
ЃЌDЕФНсЙЙМђЪНЮЊ![]() ЃЌОнДЫЗжЮіЃЛ
ЃЌОнДЫЗжЮіЃЛ
(1)ИљОнGНсЙЙМђЪНЃЌGЕФЛЏбЇУћГЦЮЊввЖўЫсЖўввѕЅЃЛAЗжзгЪНЮЊC8H9BrЃЌИљОнHНсЙЙМђЪНЃЌAжагІКЌгаБНЛЗЃЌAдкЧтбѕЛЏФЦЫЎШмвКЩњГЩBЃЌЗЂЩњТБДњЬўЕФЫЎНтЃЌєЧЛљШЁДњЃBrЕФЮЛжУЃЌBбѕЛЏГЩCбѕЛЏГЩDЃЌетЪЧДМБЛбѕЛЏГЩШЉЃЌШЉБЛбѕЛЏГЩЫсЃЌМДAЕФНсЙЙМђЪНЮЊ![]() ЃЌBЕФНсЙЙМђЪНЮЊ
ЃЌBЕФНсЙЙМђЪНЮЊ![]() ЃЌCЕФНсЙЙМђЪНЮЊ
ЃЌCЕФНсЙЙМђЪНЮЊ![]() ЃЌDЕФНсЙЙМђЪНЮЊ
ЃЌDЕФНсЙЙМђЪНЮЊ![]() ЃЌдђCЕФЗжзгЪНC8H8OЃЛ
ЃЌдђCЕФЗжзгЪНC8H8OЃЛ
(2)ИљОнFЕФЗжзгЪНЃЌвдМАGЕФНсЙЙМђЪНЃЌFгыXЗЂЩњѕЅЛЏЗДгІЃЌМДFЮЊввЖўЫсЃЌXНсЙЙМђЪНЮЊCH3CH2OHЃЛ
(3)ИљОнHЕФНсЙЙМђЪНЃЌКЌгаЕФЙйФмЭХЪЧѕЅЛљЁЂєЪЛљЃЛЖдБШIКЭJНсЙЙМђЪНЃЌC2H5BrжаЕФЃC2H5ШЁДњIжаЁАCHЁБЩЯЕФHЃЌМДIЁњJЮЊШЁДњЗДгІЃЛ
(4)ЂйКЌгаБНЛЗЃЌБНЛЗЩЯгаСНИіШЁДњЛљЃЌетСНИіШЁДњЛљЮЛжУЮЊСкЁЂМфЁЂЖдЃЛЂкФмЗЂЩњЫЎНтЗДгІЧвФмЗЂЩњвјОЕЗДгІЃЌИљОнEЕФЗжзгЪНЃЌвђДЫИУЮяжЪНсЙЙКЌгаЁА ЁБЃЌзлЩЯЫљЪіЃЌ
ЁБЃЌзлЩЯЫљЪіЃЌ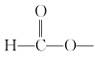 гыБНЛЗжЎМфЯрСЌЃЌСэвЛИіШЁДњЛљЮЊЃCH2CH2CH3ЁЂЃCH(CH3)3ЃЛ
гыБНЛЗжЎМфЯрСЌЃЌСэвЛИіШЁДњЛљЮЊЃCH2CH2CH3ЁЂЃCH(CH3)3ЃЛ ЭЌвЛИіCдзггыБНЛЗЯрСЌЃЌСэвЛИіШЁДњЛљЮЊЃCH2CH3ЃЛШє
ЭЌвЛИіCдзггыБНЛЗЯрСЌЃЌСэвЛИіШЁДњЛљЮЊЃCH2CH3ЃЛШє![]() гыБНЛЗжЎМфЯрСЌЃЌСэвЛИіШЁДњЛљЮЊЃCH3ЃЛШє
гыБНЛЗжЎМфЯрСЌЃЌСэвЛИіШЁДњЛљЮЊЃCH3ЃЛШє![]() гыБНЛЗжЎМфЯрСЌЃЌСэвЛИіШЁДњЛљЮЊЃCH3ЃЛзлЩЯЫљЪіЃЌЗћКЯвЊЧѓЕФЭЌЗжвьЙЙЬхЕФжжРрга3ЁС5=15жжЃЛ
гыБНЛЗжЎМфЯрСЌЃЌСэвЛИіШЁДњЛљЮЊЃCH3ЃЛзлЩЯЫљЪіЃЌЗћКЯвЊЧѓЕФЭЌЗжвьЙЙЬхЕФжжРрга3ЁС5=15жжЃЛ
(5)JЁњБНАЭБШЭзЗЂЩњШЁДњЗДгІЃЌЗДгІЗНГЬЪНЮЊ![]() +
+ +2C2H5OHЃЛ
+2C2H5OHЃЛ
(6)ИљОнКЯГЩЮяжЪЃЌвдМАJЁњБНАЭБШЭзЃЌашвЊЕФЮяжЪГ§ФђЫиЭтЃЌЛЙашвЊC2H5OOCCH2CH2COOC2H5ЃЌвђДЫЯШШУ1ЃЌ3ЃЖЁЖўЯЉгыBr2ЕФCCl4ШмвКЗЂЩњ1ЃЌ4ЃМгГЩЃЌЕУЕНCH2BrCH=CHCH2BrЃЌШЛКѓдйгыЧтЦјЗЂЩњМгГЩЗДгІЃЌЕУЕНCH2BrCH2CH2CH2BrЃЌдкNaOHЕФЫЎШмвКжаЗЂЩњЫЎНтЗДгІЃЌЕУЕНHOCH2CH2CH2CH2OHЃЌНЋЦфбѕЛЏГЩHOOCCH2CH2COOHЃЌгыввДМЗЂЩњѕЅЛЏЗДгІЃЌКЯГЩТЗЯпЮЊCH2=CHЃCH=CH2![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br ![]() HOCH2CH2CH2CH2OH
HOCH2CH2CH2CH2OH![]() HOOCCH2CH2COOH
HOOCCH2CH2COOH![]() C2H5OOCCH2CH2COOC2H5
C2H5OOCCH2CH2COOC2H5  ЁЃ
ЁЃ


 ЕМбЇШЋГЬСЗДДгХбЕСЗЯЕСаД№АИ
ЕМбЇШЋГЬСЗДДгХбЕСЗЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаБфЛЏжаЃЌЦјЬхБЛЛЙдЕФЪЧ
A.ЫЎеєЦјЪЙ CuSO4ЙЬЬхБфРЖB.ТШЦјЪЙЧГТЬЩЋ FeC12 ШмвКБфЛЦ
C.H2ЪЙзЦШШCuO ЙЬЬхБфКьD.АБЦјЪЙA1Cl3ШмвКВњЩњАзЩЋГСЕэ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАДЕк26НьЙњМЪМЦСПДѓЛсжиаТЖЈвхЃЌАЂЗќМгЕТТоГЃЪ§(NA)гаСЫзМШЗжЕ6.02214076ЁС1023ЁЃЯТСагаЙиNAЕФЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A.28gввЯЉгыБћЯЉЕФЛьКЯЦјЬхжаКЌга4NAИіC-HМќ
B.БъзМзДПіЯТЃЌ3.36LNO2жабѕдзгЪ§ФПЮЊ0.3NA
C.1L1molЁЄL-1ЕФCH3COOHШмвКгы1L0.5molЁЄL-1ЕФNaOHШмвКЛьКЯКѓЃЌCH3COO-ЕФЪ§ФПЮЊ0.5NA
D.РэТлЩЯЃЌ149gNaClOгызуСПKIШмвКЗДгІПЩЕУЕНNAИіЕтЗжзг
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПБЛгўЮЊЁАПѓЪЏамУЈЁБЕФЯуЛЈЪЏЃЌгЩЮвЙњЕижЪбЇМвЪзДЮЗЂЯжЃЌЫќгЩЧА20КХдЊЫижаЕФ6жжжїзхдЊЫизщГЩЃЌЦфЛЏбЇЪНЮЊY2X3(ZWR4)3T2ЃЌЦфжаXЁЂYЁЂZЮЊН№ЪєдЊЫиЃЌZЕФзюЭтВуЕчзгЪ§гыДЮЭтВуЕчзгЪ§ЯрЕШЃЌXЁЂZЮЛгкЭЌзхЃЌYЁЂZЁЂRЁЂTЮЛгкЭЌжмЦкЃЌRзюЭтВуЕчзгЪ§ЪЧДЮЭтВуЕФ3БЖЃЌTЮое§МлЃЌXгыRдзгађЪ§жЎКЭЪЧWЕФ2БЖЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧ( )
A.дзгАыОЖЃКY>Z>R>TB.XR2ЁЂWR2СНжжЛЏКЯЮяжаRЕФЛЏКЯМлЯрЭЌ
C.зюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЕФМюадЃКX>ZD.ЦјЬЌЧтЛЏЮяЕФЮШЖЈадЃКW<R<T
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПУКЕФзлКЯРћгУЪЧвЛИіМѕЩйЮлШОЁЂЬсИпШМСЯРћгУТЪЕФживЊПЮЬтЃЌЦфГЃгУЕФЗНЗЈАќРЈУКЕФЦјЛЏЁЂвКЛЏвдМАзЊЛЏЮЊгаЛњВњЦЗЕШЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉвбжЊЃКI.C(s)ЁЂCO(g)ЁЂH2(g)ЕФШМЩеШШвРДЮЮЊЁїH1=-393.5kJЁЄmol-1ЁЂЁїH2=-283.0kJЁЄmol-1ЁЂЁїH3=-285.8kJЁЄmol-1
II.H2O(l)=H2O(g)ЁїH4=ЃЋ44.0kJЁЄmol-1
дђУКЦјЛЏжївЊЗДгІC(s)ЃЋH2O(g)![]() CO(g)ЃЋH2(g)ЕФЁїH=___ЁЃ
CO(g)ЃЋH2(g)ЕФЁїH=___ЁЃ
ЃЈ2ЃЉЯждквЛКуЮТЕФИеадШнЦїжаМгШызуСПУКЃЌВЂЭЈШы1molЫЎеєЦјЃЌЗЂЩњЗДгІC(s)ЃЋH2O(g) ![]() CO(g)ЃЋH2(g)ЃЌдђЯТСажИБъФмЙЛЫЕУївбЕНДяЦНКтзДЬЌЕФга___ (ЬюБъКХ)ЁЃ
CO(g)ЃЋH2(g)ЃЌдђЯТСажИБъФмЙЛЫЕУївбЕНДяЦНКтзДЬЌЕФга___ (ЬюБъКХ)ЁЃ
ЂйЦјЬхЕФУмЖШБЃГжВЛБфЃЛЂкЖЯСб2molH-OМќЕФЭЌЪБЩњГЩ1molH-HМќЃЛЂлCOЕФЬхЛ§ЗжЪ§БЃГжВЛБфЃЛЂмЦјЬхЕФЦНОљФІЖћжЪСПВЛБфЃЛЂнCOгыH2ЕФБШР§БЃГжВЛБфЃЛЂоШнЦїЕФбЙЧПБЃГжВЛБфЁЃ
ЃЈ3ЃЉЦјЛЏКѓЃЌНЋЫЎУКЦјCOгыH2ЛЏКЯCO(g)ЃЋ2H2(g)![]() CH3OH(g)ПЩЕУЕНМзДМЃЌзюжеЪЕЯжУКЕФМфНгвКЛЏЁЃвбжЊдкTЁцЪБЃЌЦфе§ЗДгІЫйТЪЮЊvе§=kе§ЁЄ(CO)ЁЄc2(H2)ЃЌФцЗДгІЫйТЪЮЊvФц=kФцЁЄc(CH3OH)ЃЌЦфжаkЮЊЫйТЪГЃЪ§ЃЌЦфЪ§жЕkе§=97.5ЃЌkФц=39.0ЃЌдђИУЮТЖШЯТЕФЦНКтГЃЪ§K=___ЃЛШєдкTЁцЯТЃЌЯђвЛЬхЛ§ЮЊ2LЕФИеадУмБеЬхЯЕжаЭЈШы3molCOЁЂ2molH2КЭ5molCH3OHЃЌдђДЫЪБжЄvе§___vФц(ЬюЁАДѓгкЁБЁАаЁгкЁБЛђЁАЕШгкЁБ)ЁЃ
CH3OH(g)ПЩЕУЕНМзДМЃЌзюжеЪЕЯжУКЕФМфНгвКЛЏЁЃвбжЊдкTЁцЪБЃЌЦфе§ЗДгІЫйТЪЮЊvе§=kе§ЁЄ(CO)ЁЄc2(H2)ЃЌФцЗДгІЫйТЪЮЊvФц=kФцЁЄc(CH3OH)ЃЌЦфжаkЮЊЫйТЪГЃЪ§ЃЌЦфЪ§жЕkе§=97.5ЃЌkФц=39.0ЃЌдђИУЮТЖШЯТЕФЦНКтГЃЪ§K=___ЃЛШєдкTЁцЯТЃЌЯђвЛЬхЛ§ЮЊ2LЕФИеадУмБеЬхЯЕжаЭЈШы3molCOЁЂ2molH2КЭ5molCH3OHЃЌдђДЫЪБжЄvе§___vФц(ЬюЁАДѓгкЁБЁАаЁгкЁБЛђЁАЕШгкЁБ)ЁЃ
ЃЈ4ЃЉЙигкCO(g)ЃЋ2H2(g)![]() CH3OH(g)ЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ___(ЬюзжФИ)ЁЃ
CH3OH(g)ЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ___(ЬюзжФИ)ЁЃ
A.МгбЙгаРћгкЫйТЪМгПьКЭЦНКтЯђе§ЗДгІЗНЯђвЦЖЏ
B.МзДМЕФЦНКтЬхЛ§ЗжЪ§ЫцзХCOгыH2ЭЖСЯБШЕФдіДѓЖјдіДѓ
C.ЪЙгУДпЛЏадФмКУЕФДпЛЏМСЃЌПЩЬсИпH2ЕФЦНКтзЊЛЏТЪ
D.дкКуЮТКуШнЬѕМўЯТДяЕНЦНКтКѓЃЌЭЈШыArЃЌЦНКтЯђФцЗДгІЗНЯђвЦЖЏ
E.вбжЊE[CO(g)ЃЋ2H2(g)]>E[CH3OH(g)](EБэЪОЮяжЪЕФФмСП)ЃЌдђНЕЮТгаРћгкЬсИпе§ЗДгІНјааЕФГЬЖШ
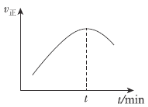
ЃЈ5ЃЉдквЛЬиЪтЕФКуШнШнЦїжаЃЌГфШывЛЖЈСПЕФCO(g)гыH2(g)РДФЃФтCO(g)ЃЋ2H2(g)![]() CH3OH(g)ЃЌВтЕУvе§ЫцЪБМфЕФБфЛЏЧњЯпШчЭМЫљЪОЃЌдђtжЎЧАvе§ж№НЅдіДѓЕФдвђЮЊ___ЃЛtжЎКѓvе§гжж№НЅМѕаЁЕФдвђЮЊ___ЁЃ
CH3OH(g)ЃЌВтЕУvе§ЫцЪБМфЕФБфЛЏЧњЯпШчЭМЫљЪОЃЌдђtжЎЧАvе§ж№НЅдіДѓЕФдвђЮЊ___ЃЛtжЎКѓvе§гжж№НЅМѕаЁЕФдвђЮЊ___ЁЃ
ЃЈ6ЃЉУКОЙ§вЛЯЕСазЊЛЏЛЙПЩЕУЕНВнЫсЁЃГЃЮТЯТЃЌЯђФГХЈЖШЕФВнЫсШмвКжаМгШывЛЖЈСПФГХЈЖШЕФNaOHШмвКЃЌЫљЕУШмвКжаc(HC2O4-)=c(C2O42-)ЃЌдђДЫЪБШмвКЕФpH=___(вбжЊГЃЮТЯТH2C2O4ЕФKa1=6ЁС10-2ЃЌKa2=6ЁС10-5ЃЌlg6=0.8)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЃКAЪЧЪЏгЭСбНтЦјЕФжївЊГЩЗнЁЃЯжвдAЮЊжївЊдСЯКЯГЩввЫсввѕЅЃЌЦфКЯГЩТЗЯпШчЯТЭМЫљЪОЁЃЛиД№ЯТСаЮЪЬтЃК
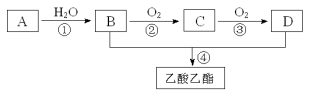
ЃЈ1ЃЉAзЊЛЏЮЊBЕФЗДгІРраЭ___________ЁЃ
ЃЈ2ЃЉDЗжзгжаЙйФмЭХЕФУћГЦ_______ЁЃ
ЃЈ3ЃЉаДГіBгыН№ЪєФЦЗДгІЕФЛЏбЇЗНГЬЪНЪЧ____________________ЁЃ
ЃЈ4ЃЉЯТСаЫЕЗЈе§ШЗЕФЪЧ________________ЁЃ
AЃЎBЗжзгжаЕФЫљгадзгдкЭЌвЛЦНУцЩЯ
BЃЎвЛЖЈЬѕМўЯТЃЌCжаЕФЙйФмЭХПЩвдгУвјАБШмвКРДМьбщ
CЃЎAПЩвддкЦфЫћЬѕМўЯТБЛбѕЦјбѕЛЏГЩввЫс
DЃЎAЪЙЫсадKMnO4ШмвКЭЪЩЋКЭЪЙфхЫЎЭЪЩЋЃЌЦфдРэЯрЭЌ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПУОЁЊЬрвКЬЌН№ЪєДЂФмЕчГиЙЄзїдРэШчЯТЭМЫљЪОЃЌИУЕчГиЫљгУвКЬхУмЖШВЛЭЌЃЌдкжиСІзїгУЯТЗжЮЊШ§ВуЃЌЙЄзїЪБжаМфВуШлШкбЮЕФзщГЩМАХЈЖШВЛБфЁЃЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
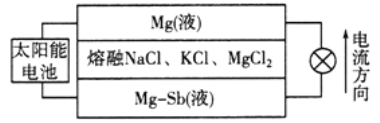
A. ЗХЕчЪБЃЌMg(вК)ВуЕФжЪСПМѕаЁ
B. ЗХЕчЪБЃЌе§МЋЗДгІЮЊЃКMg2++2e===Mg
C. ИУЕчГиГфЕчЪБЃЌMgЁЊSb(вК)ВуЕФжЪСПдіДѓ
D. ИУЕчГиГфЕчЪБЃЌC1ЯђЯТВуЗНЯђвЦЖЏ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПСзЫсТШрдкЯИАћЫЎЦНЩЯФмгааЇвжжЦаТаЭЙкзДВЁЖО2019-nCoVЕФИаШОЁЃЦфжажжвдЗМЯуЛЏКЯЮяA КЭввѕЃввЫсввѕЅ(![]() )ЮЊдСЯКЯГЩСзЫсТШрЕФКЯГЩТЗЯпШчЭМЃК
)ЮЊдСЯКЯГЩСзЫсТШрЕФКЯГЩТЗЯпШчЭМЃК
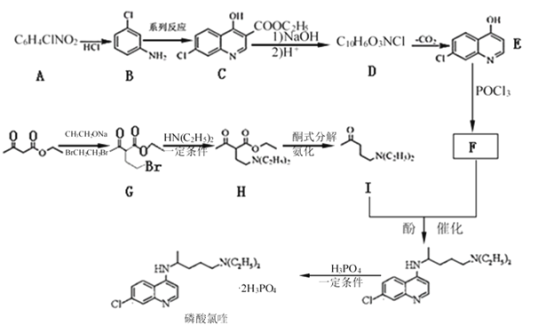
вбжЊЃКЂй![]() ОпгаЫсад
ОпгаЫсад
Ђк![]()
![]()
![]()
ЂлR1NH2+R2X![]() R1NHR2+HX(XБэЪОТБдзг)ЁЃ
R1NHR2+HX(XБэЪОТБдзг)ЁЃ
ЧыЛиД№ЯТСаЮЪЬтЃК
(1) EжаКЌбѕЙйФмЭХЕФУћГЦЪЧ________ЃЌGЁњHЕФЗДгІРраЭЪЧ________ЁЃ
(2)ЬМдзгЩЯСЌга4ИіВЛЭЌЕФдзгЛђЛљЭХЪБЃЌИУЬМГЦЮЊЪжадЬМЁЃаДГіBгызуСПЕФЧтЦјМгГЩКѓЕФВњЮяMЕФНсЙЙМђЪНЃЌгУаЧКХ(*)БъГіMжаЕФЪжадЬМ_________ЁЃ
(3) FЕФНсЙЙМђЪНЪЧ_______ЁЃ
(4)аДГіCгыNaOHЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___________ЁЃ
(5) NЪЧHЕФЭЌЗжвьЙЙЬхЃЌЗћКЯЯТСаЬѕМўЕФNЕФЭЌЗжвьЙЙЬхЙВга____жжЃК аДГіЦфжавЛжжКЫДХЙВеёЧтЦзЯдЪОЛЗЩЯжЛга3зщЗхЃЌЧвЗхУцЛ§жЎБШЮЊ4ЃК4ЃК1ЕФНсЙЙМђЪН________ЁЃ
ЂйКЌгаЛљЭХ![]() ЁЂ-N(C2H5)2
ЁЂ-N(C2H5)2
ЂкЫсадЫЎНтВњЮяжаКЌгаввЫс
ЂлФмгыН№ЪєФЦЗДгІЩњГЩH2
(6)ВЮееЩЯЪіаХЯЂКЭКЯГЩТЗЯпЃЌЧыЩшМЦвдБНКЭ1ЃЌ4 -ЖЁЖўДМЮЊдСЯКЯГЩ![]() ЕФКЯГЩТЗЯп(ЮоЛњЪдМСШЮбЁ)____________ЁЃ
ЕФКЯГЩТЗЯп(ЮоЛњЪдМСШЮбЁ)____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПНЋ43.2g FeC2O4ИєОјПеЦјМгШШЃЌЗжНтЕУЕН21.6gЬњЕФбѕЛЏЮяЃЌЫљЕУЦјЬхВњЮяГЩЗжМАЮяжЪЕФСПЪЧ
A.0.3mol COB.0.3mol COКЭ0.3mol CO2
C.0.6mol CO2D.0.5mol COКЭ0.5mol CO2
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com