
����Ŀ���������ǵ�����ͻ�ѧ����ʱʹ�õ���Ҫ���Ρ��Է�����������Ҫ�ɷ�ΪNiCO3��SiO2������������Fe2O3��Cr2O3��Ϊԭ���Ʊ������������������ͼ��ʾ��
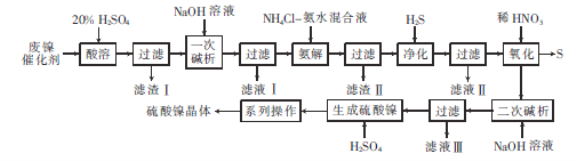
��֪��Ni3+�������Ա�ϡHNO3ǿ��
�ش��������⣺
(1)��֪��Cr3++4OH-= CrO2-+2H2O����һ�μ�����ʱ������������NaOH��Һ��Ŀ����______________________��
(2)����������Ŀ��Ϊ_______________________��
(3)������������������õ���������Ԫ�صIJ����Ի�����û�����Ļ�ѧʽΪ________��д�����������з�����Ӧ�����ӷ���ʽ��____________________________________��
(4)��NiSO4��Һ�еõ������������辭����ϵ�в�����Ϊ__________��__________�����ˣ�ϴ�ӣ������������ƣ���
(5)1844�꣬��ѧ�ҷ��ֽ�����������NaH2PO2��ˮ��Һ�е�Ni2+��ԭ������NaH2PO2��ת��ΪH3PO3����һԭ�������ڻ�ѧ������д����ѧ����ԭ�������ӷ���ʽ___________��
(6)Ϊ�ⶨ���������壨NiSO4��n H2O������ɣ���������ʵ�飺��ȡ2.63g��Ʒ�����250.00mL��Һ��ȷ��ȡ���Ƶ���Һ25.00mL����0.0400mol/L EDTA(Na2H2Y)����Һ�ζ�Ni2+�����ӷ���ʽΪNi2++H2Y2-=NiY2-+2H+��������EDTA����Һ25.00mL��������������Ļ�ѧʽΪ_____________________________��
���𰸡���ȥCrԪ�� ʵ����Ԫ������Ԫ�صķ��� NiS 3NiS+8H++2NO3-=3Ni2++2NO��+3S��4H2O ����Ũ�� ��ȴ�ᾧ Ni2++H2PO2-+H2O=H3PO3+H++Ni NiSO4�q6H2O
��������
������������Ҫ�ɷ�ΪNiCO3��SiO2������������Fe2O3��Cr2O3������ʱNiCO3��Fe2O3��Cr2O3�����ᷴӦ����NiSO4��Fe2(SO4)3��Cr2(SO4)3��SiO2����Ӧ�����˺���Һ�м���NaOH����һ�μ��������ݡ�Cr3++4OH-= CrO2-+2H2O����NiSO4��Fe2(SO4)3ת����Ni��OH��2��Fe��OH��3������CrԪ��ת����CrO2-������ҺI�У����������Ni��OH��2ת����[Ni(NH3)6]2+������H2S����ʱ[Ni(NH3)6]2+ת��ΪNiS������NiS�м���ϡ���ᣬ����Ni3+�������Ա�ϡHNO3ǿ����ϡ���ὫNiSת����Ni(NO3)2��S�����μ���ʱNi(NO3)2��NaOH��Ӧ����Ni��OH��2������Ni��OH��2�����ᷴӦ����NiSO4����������Һ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���������������壻�ݴ˷�������
��1�����ݡ�Cr3++4OH-= CrO2-+2H2O������һ�μ�����ʱ���������NaOH��Һ����Cr3+����������Ӧ������CrO2-����ȥ��CrԪ�أ��ʱ���𰸣���ȥCrԪ�ء�
��2����������м���NH4Cl����ˮ���Һ��Ni��OH��2ת����[Ni(NH3)6]2+�������ܽ�Fe��OH��3���Ӷ�ʵ����Ԫ�غ���Ԫ�صķ��룬�����Ϊ��ʵ����Ԫ�غ���Ԫ�صķ��롣
��3����������������ͨ��H2S������NiS�������������������У���ΪNi3+�������Ա�ϡHNO3ǿ��ϡHNO3����ԭΪNO��NiS������ΪS����Ӧ�����ӷ���ʽΪ3NiS+8H++2NO3-=3Ni2++2NO��+3S��4H2O���ʱ����Ϊ��NiS��3NiS+8H++2NO3-=3Ni2++2NO��+3S��4H2O��
��4����NiSO4��Һ�еõ������������辭����ϵ�в�����Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ʱ����Ϊ������Ũ������ȴ�ᾧ��
��5����NaH2PO2��ˮ��Һ�е�Ni2+��ԭ������NaH2PO2��ת��ΪH3PO3��NiԪ�صĻ��ϼ���+2�۽�Ϊ0�ۣ�PԪ�صĻ��ϼ���+1������+3�ۣ����ݵ�ʧ�����غ㡢ԭ���غ�͵���غ㣬�����ӷ���ʽΪ��Ni2++H2PO2-+H2O=H3PO3+H++Ni���ʱ����Ϊ��Ni2++H2PO2-+H2O=H3PO3+H++Ni��
��6�����ݷ���ʽNi2++H2Y2-=NiY2-+2H+��֪��ԭ��Ʒ�к�NiSO4�����ʵ���Ϊ��0.0400mol/L��0.025L��10=0.01mol�������ʵĵ�Ħ������Ϊ![]() =263g/mol���ᾧˮ��ϵ��nΪ
=263g/mol���ᾧˮ��ϵ��nΪ![]() =6����ѧʽΪ��NiSO4�q6H2O�������Ϊ��NiSO4�q6H2O��
=6����ѧʽΪ��NiSO4�q6H2O�������Ϊ��NiSO4�q6H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.1000 mol��L��1 NaOH��Һ�ֱ�ζ�0.1000 mol��L��1 HA��HD��ĵζ�������ͼ��ʾ������˵������ȷ����
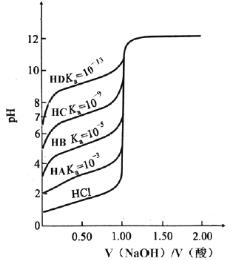
A.�����ʵ���Ũ��ʱ��KaԽС�ζ�ͻ��Խ�����ԣ��ζ����Խ��
B.�ζ�HCʱ���ζ��յ��pH��7
C.�ζ�HBʱ��Ӧ��ѡ���̪��Ϊָʾ��
D.��ͼ���֪�ڼ���HD�ζ�ǰ��ҺpHʱ���ܺ���ˮ�ĵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ǻϳ�ҩ����������м���J��һ�ֺϳ�·�ߣ�
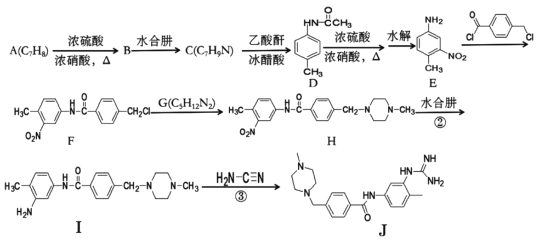
�ش��������⣺
��1��A������Ϊ____��B�Ļ�ѧʽΪ____��
��2��F�к�������������Ϊ____
��3��B��C��I��J�ķ�Ӧ���ͷֱ�Ϊ____��____��
��4��G�Ľṹ��ʽΪ____��
��5�����㻯����X��D��ͬ���칹�壬�����Ͼ�������ȡ�����Ұ����뱽��ֱ��������X�ܷ���������Ӧ�����ӽṹֻ��һ���һ�������������X�Ľṹʽ��____�֡�
��6��д���üױ����Ҵ�Ϊԭ���Ʊ����������������ĺϳ�·�ߣ�_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ������У�һ�������£��������·�Ӧ��NO(g)��CO(g) ![]()
![]() N2(g)+CO2(g)����H= -373.2kJ/mol���ﵽƽ���Ϊ��߸÷�Ӧ�����ʺ�NO��ת���ʣ���ȡ����ȷ��ʩ��
N2(g)+CO2(g)����H= -373.2kJ/mol���ﵽƽ���Ϊ��߸÷�Ӧ�����ʺ�NO��ת���ʣ���ȡ����ȷ��ʩ��
A.�Ӵ���ͬʱ�����¶�B.�Ӵ���ͬʱ����ѹǿ
C.�����¶�ͬʱ����N2D.�����¶�ͬʱ����ѹǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������CO��H2�ڴ����������ºϳɼ״��������ķ�Ӧ���£�CO(g)+2H2(g)![]() CH3OH(g)�������һ�����ܱ������а����ʵ���֮��1��2����CO��H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��ʾ������˵����ȷ����
CH3OH(g)�������һ�����ܱ������а����ʵ���֮��1��2����CO��H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��ʾ������˵����ȷ����

A. �÷�Ӧ����H��0����p1��p2
B. ��Ӧ���ʣ�����(״̬A)������(״̬B)
C. ��C��ʱ��COת����Ϊ75%
D. �ں��º�ѹ���������ܱ������г��벻ͬ����CH3OH����ƽ��ʱCH3OH���������Ҳ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ���������Ⱦ������Ҫ��ǿ�Թ�ҵ����������β����������������ѧ֪ʶ�ش��������⣺
(1)��ʯȼ�ϰ���ú��ʯ�ͺ�________��
(2)������ָpH____(����>����<������=��)5.6�Ľ�ˮ��ú��ȼ���ǵ��������γɵ���Ҫԭ��������ˮ��pHԼΪ5.6��ԭ����__________________ (�û�ѧ����ʽ��ʾ)��
(3)ú�������Ǹ�Ч���������ú����Ҫ;�����ɽ�ú���ɽ�̿���ٽ���̿�ڸ�������ˮ������Ӧ����һ����̼�������Ļ�ѧ����ʽΪ_________���÷�Ӧ�Ļ���������_________��
(4)������β���ŷſڼ�װ����Ч�������������ڲ������������ʵ�����£��ɽ�β���е�һ����̼��һ������ת��Ϊ�������ѭ����������������壬�÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
(5)������Դ����δ�ռ�ʱ����ͼ��ʾΪһ�������ܼ���������Ч��ʩ�����¶��Ҵ�������ȼ�ϵ������������__________(����ĸ)��
![]()
A.ԭ����Դ�ḻ B.�ǿ�������Դ C.ȼ����ȫû����Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)������ͼװ�ã�����NH3�����������MxOy��Ӧ����M��N2��H2O��ͨ����������ˮ���������ⶨM�����ԭ��������a���Լ���Ũ��ˮ��

������a������Ϊ_________������b��װ����Լ�������___________��
�ڰ�����������ȷ��װ������˳��Ϊ_________(����ĸ��װ�ÿ��ظ�ʹ��)��
(2)����������һ�ֹ�ҵ�Σ���������������Ӧ�ù㷺��������ͼ��ʾװ��(�г�װ����ʡ��)��ҩƷ��̽���������������ᷴӦ���������ɷ֡�

��֪����.NO+NO2+2OH-=2NO2-+H2O
��.����Һ�����¶ȣ�NO2(21��)��NO(-152��)
�ٷ�ӦǰӦ���ɼУ���ͨ��һ��ʱ�䵪����Ŀ����________________��
��Ϊ�˼���װ��A�����ɵ�����������������˳��(������������)��A��_________����װ���������������еIJ�����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС��ͬѧͨ��ʵ���о�FeCl3��Һ��Cu�۷�����������ԭ��Ӧ��ʵ���¼���£�
��� | I | II | III |
ʵ�鲽�� |
���������2mL����ˮ |
���������2mL����ˮ |
���������2mL����ˮ |
ʵ������ | ͭ����ʧ����Һ��ɫ��dz����������ˮ������������ | ͭ����ʣ�࣬��Һ��ɫ��ȥ����������ˮ�����ɰ�ɫ���� | ͭ����ʣ�࣬��Һ��ɫ��ȥ�������ɫ����������ˮ���ް�ɫ���� |
����˵������ȷ����
A. ʵ��I��II��III�о��漰Fe3+����ԭ
B. �Ա�ʵ��I��II˵����ɫ�����IJ�����ͭ�۵����й�
C. ʵ��II��III�м�������ˮ��c(Cu2+)��ͬ
D. ��ʵ��III��Ӧ�����Һ�м��뱥��NaCl��Һ���ܳ��ְ�ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����( )
A.ԭ������֮��Ϊ2������Ԫ�ز�����λ��ͬһ����
B.D-������36������ ,��Ԫ��Dλ�ڵ������ڵڢ�A��
C.λ��ͬһ�������ڵļס�������Ԫ��,��ԭ������Ϊ![]() ,���ҵ�ԭ����������Ϊ
,���ҵ�ԭ����������Ϊ![]()
D.λ��ͬһ���ڵļס�������Ԫ��,��λ�ڵ�IIA��,ԭ������Ϊ![]() ,��λ�ڵڢ�A��,����ԭ����������Ϊ
,��λ�ڵڢ�A��,����ԭ����������Ϊ![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com