
���� ��1��������NaHSO4��Һ�μӵ�Ba��OH��2��Һ�У��������ᱵ���������ƺ�ˮ��
��Ca��HCO3��2��Һ�еμӹ���NaOH��Һ����̼��ơ�̼���ƺ�ˮ��
��2��NH4HCO3��Һ������NaOH��Һ��Ӧ����һˮ�ϰ���̼���ƺ�ˮ��
��FeI2��Һ�У��μ�����ϡ���ᣬ�����ӱ�����Ϊ�ⵥ�ʣ����ᱻ��ԭΪNO��
��3���ٶ����������л�ԭ�ԣ�Cr2O72-��ǿ�����ԣ������ܷ���������ԭ��Ӧ�����ݵ��ӵ�ʧ��ƽ��
�ڴ������ƾ���ǿ�����ԣ�������I-���ɵ���I2��
��Na2SO3���л�ԭ�ԣ��ɻ�ԭI2����I-��ʹ��ɫ��ʧ��
��������ԭ��Ӧ�У������������Դ�����������������ԣ�
��� �⣺��1��������NaHSO4��Һ�μӵ�Ba��OH��2��Һ�У��������ᱵ���������ƺ�ˮ���������ӷ���ʽΪ��Ba2++SO42-+OH-+H+=BaSO4��+H2O��
��Ca��HCO3��2��Һ�еμӹ���NaOH��Һ����̼��ơ�̼���ƺ�ˮ���������ӷ���ʽΪ��Ca2++2HCO3-+2OH-+=CaCO3��+CO32-+2H2O��
�ʴ�Ϊ��Ba2++SO42-+OH-+H+=BaSO4��+H2O��Ca2++2HCO3-+2OH-+=CaCO3��+CO32-+2H2O��
��2��NH4HCO3��Һ������NaOH��Һ��Ӧ����һˮ�ϰ���̼���ƺ�ˮ�����ӷ���ʽΪNH4++HCO3-+2OH-=NH3��H2O+CO32-+H2O��
��FeI2��Һ�У��μ�����ϡ���ᣬ�����ӱ�����Ϊ�ⵥ�ʣ����ᱻ��ԭΪNO���䷴Ӧ���ӷ���ʽΪ��6I-+2NO3-+8H+�T3I2+2NO��+4H2O��
�ʴ�Ϊ��NH4++HCO3-+2OH-=NH3��H2O+CO32-+H2O��6I-+2NO3-+8H+�T3I2+2NO��+4H2O��
��3���ٶ����������л�ԭ�ԣ�Cr2O72-��ǿ�����ԣ������ܷ���������ԭ��Ӧ�����������ӱ����������������ӣ�Cr2O72-����ԭΪCr3+����Ӧ����ʽΪ��Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��
�ʴ�Ϊ��Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��
�ڴ������ƾ���ǿ�����ԣ�������I-���ɵ���I2��I2�����۱�������Ӧ�����ӷ���ʽΪClO-+2I-+H2O�TI2+Cl-+2OH-��
�ʴ�Ϊ��ClO-+2I-+H2O�TI2+Cl-+2OH-��
��Na2SO3���л�ԭ�ԣ��ɻ�ԭI2����I-��ʹ��ɫ��ʧ����Ӧ�����ӷ���ʽΪSO32-+I2+2OH-�TSO42-+2I-+H2O��
�ʴ�Ϊ��SO32-+I2+2OH-�TSO42-+2I-+H2O��
��������ԭ��Ӧ�У������������Դ�����������������ԣ��ɢٿ�֪ClO-��I2���ɢڿ�֪I2��SO42-��
�ʴ�Ϊ��ClO-��I2��SO42-��
���� ���⿼�������ӷ���ʽ����д��������ԭ��Ӧ����Ŀ�ѶȲ�����ȷ������Ӧ��ʵ��Ϊ���ؼ���ע�������������ӷ���ʽ����дԭ������������ѧ�������Ӧ��������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
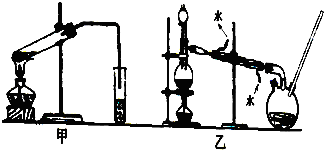
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ȶ� | B�� | �Ȼ���������ȶ� | ||
| C�� | �������Ա�������ǿ | D�� | ���������Աȸ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���³�ѹ�£�0.5molH2�к��е�Hԭ���� | |
| B�� | 22.4��HCl�����к��е�Clԭ�� | |
| C�� | 1L1mol/L������Һ������H+�� | |
| D�� | 0.1mol���������еĵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 T��ʱ����6mol CO2��8mol H2����2L�ܱպ��������У�������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����������H2�����ʵ�����ʱ��仯��ͼ��ʵ����ʾ��ͼ�����߱�ʾ���ı�ijһ��Ӧ����ʱ��H2�����ʵ�����ʱ��ı仯������˵����ȷ���ǣ�������
T��ʱ����6mol CO2��8mol H2����2L�ܱպ��������У�������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����������H2�����ʵ�����ʱ��仯��ͼ��ʵ����ʾ��ͼ�����߱�ʾ���ı�ijһ��Ӧ����ʱ��H2�����ʵ�����ʱ��ı仯������˵����ȷ���ǣ�������| A�� | ��Ӧ��ʼ��a��ʱv��H2��=2 mol/��L•min�� | |
| B�� | �����ߢ��Ӧ�������ı������£���÷�Ӧ���ȷ�Ӧ | |
| C�� | ���ߢ��Ӧ�������ı��ǽ���ѹǿ | |
| D�� | ���ߢ��Ӧ�������ı��Ǽ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
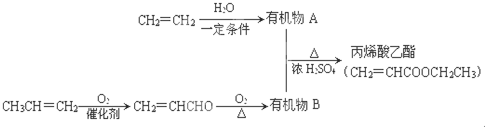
 ����ϩ�����������еĺ���������������̼̼˫����������
����ϩ�����������еĺ���������������̼̼˫���������� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
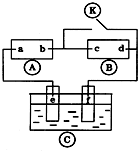 ��ͼ
��ͼ Ϊֱ����Դ��
Ϊֱ����Դ�� Ϊ�������Ȼ�����Һ�ͷ�̪��Һ����ֽ��
Ϊ�������Ȼ�����Һ�ͷ�̪��Һ����ֽ�� Ϊ��Ʋۣ���ͨ��·����
Ϊ��Ʋۣ���ͨ��·���� �ϵ�c���Ժ�ɫ��Ϊʵ�����϶�п����ͨ
�ϵ�c���Ժ�ɫ��Ϊʵ�����϶�п����ͨ ��ʹc��d�����·������������ȷ���ǣ�������
��ʹc��d�����·������������ȷ���ǣ�������| A�� | aΪֱ����Դ�ĸ��� | B�� | c�������ķ�ӦΪ2H++2e-=H2�� | ||
| C�� | f�缫Ϊп�� | D�� |  ����Һ������ΪFeSO4 ����Һ������ΪFeSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2��NH3 | B�� | N2��O2 | C�� | CO��CO2 | D�� | H2S��HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com