
ЁОЬтФПЁПЂёЃЎгаЛњЮяЕФБэЪОЗНЗЈЖржжЖрбљЃЌЯТУцЪЧГЃгУЕФгаЛњЮяЕФБэЪОЗНЗЈЃК
Ђй![]() ЂкCH3CH2CH(CH3)CH3ЂлCH4 Ђм
ЂкCH3CH2CH(CH3)CH3ЂлCH4 Ђм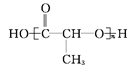 Ђн
Ђн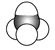
Ђо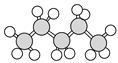 Ђп
Ђп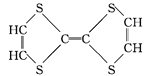 Ђр
Ђр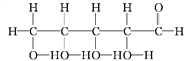
ЃЈ1ЃЉЩЯЪіБэЪОЗНЗЈжаЪєгкНсЙЙМђЪНЕФЪЧ_______________(ЬюађКХЃЌЯТЭЌ)ЃЌЪєгкБШР§ФЃаЭЕФЪЧ_______ЁЃ
ЃЈ2ЃЉаДГіЂржаЙйФмЭХЕФУћГЦЃК____________ЁЂ____________ЁЃ
ЃЈ3ЃЉ____________КЭ____________ЛЅЮЊЭЌЗжвьЙЙЬхЁЃ
ЂђЃЎЃЈ1ЃЉаДГіЯТСагаЛњЮяЕФНсЙЙМђЪНЃК
Ђй2ЃЌ4ЖўМзЛљ3ввЛљМКЭщ____________ЃЛ
Ђк2МзЛљ2ЮьЯЉ____________ЁЃ
ЃЈ2ЃЉЖдЯТСагаЛњЮяНјааУќУћЃК
Ђй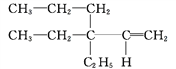 ____________ЁЃ
____________ЁЃ
Ђк(CH3)3CCH(CH3)CH2CH3____________ЁЃ
ЁОД№АИЁПЂйЂкЂлЂмЂп Ђн єЧЛљ ШЉЛљ Ђк Ђо 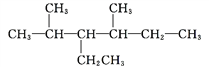
![]() 3ЃЌ3ЖўввЛљ1МКЯЉЁЁ 2ЃЌ2ЃЌ3Ш§МзЛљЮьЭщ
3ЃЌ3ЖўввЛљ1МКЯЉЁЁ 2ЃЌ2ЃЌ3Ш§МзЛљЮьЭщ
ЁОНтЮіЁП
ЂёЃЎЃЈ1ЃЉЂйЂкЂлЂмЂпЪєгкгаЛњЮяЕФНсЙЙМђЪНЃЌЂнЪєгкгаЛњЮяЕФБШР§ФЃаЭЃЌЂоЪєгкгаЛњЮяЕФЧђЙїФЃаЭЃЌЂрЪєгкгаЛњЮяЕФНсЙЙЪНЃЛ
ЃЈ2ЃЉзЂвтЙйФмЭХгаСНжжЃКЃOHЪЧєЧЛљЃЌ![]() ЪЧШЉЛљЃЛ
ЪЧШЉЛљЃЛ
ЃЈ3ЃЉЂкЂоЕФЗжзгЪНЖМЪЧC5H12ЃЌЕЋНсЙЙВЛЭЌЃЌЪєгкЭЌЗжвьЙЙЬхЃЛ
ЂђЃЎЃЈ1ЃЉЯШаДГіжїСДЃЌдйаДжЇСДЁЃ2ЃЌ4ЃЖўМзЛљЃ3ЃввЛљМКЭщЕФНсЙЙМђЪНЮЊ ЃЌ2МзЛљ2ЮьЯЉЃЌПЩвдЯШаДГіCЃC
ЃЌ2МзЛљ2ЮьЯЉЃЌПЩвдЯШаДГіCЃC![]() CЃCЃCЃЌдйдк2КХЬМдзгЩЯв§ШыМзЛљЃЌзюКѓАбЧтдзгВЙЦыЃЌМДНсЙЙМђЪНЮЊ
CЃCЃCЃЌдйдк2КХЬМдзгЩЯв§ШыМзЛљЃЌзюКѓАбЧтдзгВЙЦыЃЌМДНсЙЙМђЪНЮЊ![]() ЃЛ
ЃЛ
ЃЈ2ЃЉЂйКЌгаЬМЬМЫЋМќЕФзюГЄЬМСДКЌга6ИіЬМдзгЃЌУћГЦЪЧ3ЃЌ3ЃЖўввЛљЃ1ЃМКЯЉЃЛ
ЂкНЋКЯВЂЕФНсЙЙМђЪНИФаДГЩЗжПЊЕФНсЙЙМђЪНЃК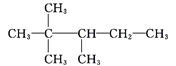 ЃЌвђДЫУћГЦЪЧ2ЃЌ2ЃЌ3ЃШ§МзЛљЮьЭщЁЃ
ЃЌвђДЫУћГЦЪЧ2ЃЌ2ЃЌ3ЃШ§МзЛљЮьЭщЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЦЋЖўМзыТгыN2O4ЪЧГЃгУЕФЛ№М§ЭЦНјМСЃЌЖўепЗЂЩњШчЯТЛЏбЇЗДгІЃК
(Ђё)(CH3)2NNH2(l)+2N2O4(l)=2CO2(g)+3N2(g)+4H2O(g)
(Ђђ)N2O4(g)2NO2(g)
ЃЈ1ЃЉЗДгІ(Ђё)жабѕЛЏМСЪЧ___ЁЃ
ЃЈ2ЃЉЕБЮТЖШЩ§ИпЪБЃЌЦјЬхбеЩЋБфЩюЃЌдђЗДгІ(Ђђ)ЮЊ___(ЬюЁАЮќШШЁБЛђЁАЗХШШЁБ)ЗДгІЁЃ
ЃЈ3ЃЉШєдкЯрЭЌЮТЖШЯТЃЌЩЯЪіЗДгІИФдкЬхЛ§ЮЊ1LЕФКуШнУмБеШнЦїжаНјааЃЌЦНКтГЃЪ§___(ЬюЁАдіДѓЁБЁАВЛБфЁБЛђЁАМѕаЁЁБ)ЃЌЗДгІ3sКѓNO2ЕФЮяжЪЕФСПЮЊ0.6molЃЌдђ0ЁЋ3sФкЕФЦНОљЗДгІЫйТЪv(N2O4)=_______molL-1s-1ЁЃ
ЃЈ4ЃЉNO2ПЩгУАБЫЎЮќЪеЩњГЩNH4NO3ЁЃ25ЁцЪБЃЌНЋamolNH4NO3ШмгкЫЎЃЌШмвКЯдЫсадЃЌдвђЪЧ___(гУРызгЗНГЬЪНБэЪО)ЁЃ
ЃЈ5ЃЉЯђИУШмвКЕЮМгbLАБЫЎКѓШмвКГЪжаадЃЌдђЕЮМгАБЫЎЕФЙ§ГЬжаЫЎЕФЕчРыЦНКтНЋ___(ЬюЁАе§ЯђЁБЁАВЛЁБЛђЁАФцЯђЁБ)вЦЖЏЃЌЫљЕЮМгАБЫЎЕФХЈЖШЮЊ___molЁЄL-1ЁЃ(NH3ЁЄH2OЕФЕчРыЦНКтГЃЪ§ШЁKb=2ЁС10-5molЁЄL-1ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСабѕЛЏЛЙдЗДгІЗНГЬЪНЃЌ ЫљБъЕчзгзЊвЦЗНЯђгыЪ§ФПДэЮѓЕФЪЧЃЈ ЃЉ
A.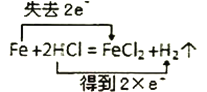 B.
B.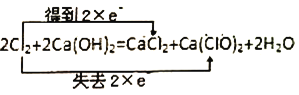
C.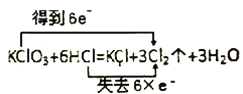 D.
D.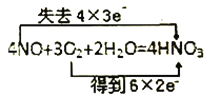
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУШчЭМЫљЪОЪЕбщзАжУНјааЮяжЪаджЪЕФЬНОПЪЕбщЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧЃЈ ЃЉ
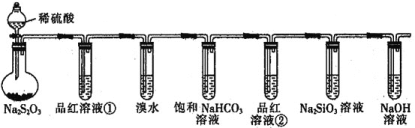
A.ШєЦЗКьШмвКЂйЭЪЩЋЃЌдђЫЕУїВњЮяжаКЌгаSO2
B.ШєфхЫЎЭЪЩЋЃЌдђЫЕУїSO2ОпгаЛЙдад
C.ШєЩеЦПжаГіЯжЕЛЦЩЋЛызЧКЭЮоЩЋЦјХнЃЌдђЫЕУїNa2S2O3жЛзїбѕЛЏМС
D.ШєЦЗКьШмвКЂкВЛЭЪЩЋЃЌNa2SiO3ШмвКГіЯжАзЩЋЛызЧЃЌдђЫЕУїбЧСђЫсБШЬМЫсЫсадЧП
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПT KЪБЃЌЯђ2.0 LКуШнУмБеШнЦїжаГфШы1.0 mol COCl2ЃЌЗДгІCOCl2(g)![]() Cl2(g)ЃЋCO(g)ЃЌОЙ§вЛЖЮЪБМфКѓДяЕНЦНКтЁЃЗДгІЙ§ГЬжаВтЖЈЕФВПЗжЪ§ОнМћЯТБэЃК
Cl2(g)ЃЋCO(g)ЃЌОЙ§вЛЖЮЪБМфКѓДяЕНЦНКтЁЃЗДгІЙ§ГЬжаВтЖЈЕФВПЗжЪ§ОнМћЯТБэЃК
t / s | 0 | 2 | 4 | 6 | 8 |
n(Cl2) / mol | 0 | 0.16 | 0.19 | 0. 20 | 0.20 |
ЯТСаЫЕЗЈе§ШЗЕФЪЧ( )
A. ЗДгІдкЧА2 s ЕФЦНОљЫйТЪv(CO)=0.080molЁЄL-1ЁЄs-1
B. БЃГжЦфЫћЬѕМўВЛБфЃЌЩ§ИпЮТЖШЃЌЦНКтЪБc(Cl2) =0.11molЁЄL-1ЃЌдђЗДгІЕФІЄHЃМ0
C. T KЪБЦ№ЪМЯђШнЦїжаГфШы0.9 mol COCl2ЁЂ0.10 mol Cl2КЭ0.10 mol COЃЌДяЕНЦНКтЧАvе§>vФц
D. T KЪБЦ№ЪМЯђШнЦїжаГфШы1.0 mol Cl2КЭ0.9 mol COЃЌДяЕНЦНКтЪБЃЌCl2ЕФзЊЛЏТЪЮЊ80%
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙЄвЕЩЯРћгУПЩФцЗДгІN2(g)ЃЋ3H2(g) ![]() 2NH3(g)КЯГЩАБЃЌЯТСаа№Ъіе§ШЗЕФЪЧ( )
2NH3(g)КЯГЩАБЃЌЯТСаа№Ъіе§ШЗЕФЪЧ( )
AЃЎКЯГЩАБЕФЪЪвЫЬѕМўЪЧИпЮТИпбЙДпЛЏМСЃЌЦфжаДпЛЏМСВЛИФБфИУЗДгІЕФФцЗДгІЫйТЪ
BЃЎКуШнЭЈШыыВЦјЃЌЪЙЗДгІЬхЯЕЕФбЙЧПдіДѓЃЌЗДгІЫйТЪвЛЖЈдіДѓ
CЃЎИУЗДгІЪЧЗХШШЗДгІЃЌНЕЕЭЮТЖШНЋЫѕЖЬЗДгІДяЕНЦНКтЕФЪБМф
DЃЎдкt1ЁЂt2ЪБПЬЃЌNH3 (g)ЕФХЈЖШЗжБ№ЪЧc1ЁЂc2ЃЌдђЪБМфМфИєt1ЁЋt2ФкЃЌNH3 (g)ЩњГЩЕФЦНОљЫйТЪЮЊvЃН![]()
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪЕбщЪвжаФГаЉЦјЬхЕФжЦШЁЁЂЪеМЏЁЂЮВЦјДІРэ![]() ЛђаджЪЪЕбщ
ЛђаджЪЪЕбщ![]() зАжУШчЭМЫљЪОЃЌгУДЫзАжУКЭЯТБэжаЬсЙЉЕФЮяжЪЭъГЩЯрЙиЪЕбщЃЌКЯРэЕФбЁЯюЪЧ
зАжУШчЭМЫљЪОЃЌгУДЫзАжУКЭЯТБэжаЬсЙЉЕФЮяжЪЭъГЩЯрЙиЪЕбщЃЌКЯРэЕФбЁЯюЪЧ![]()
бЁЯю | ЂёжаЕФЮяжЪ | ЂђжаЪеМЏЕФЦјЬх | ЂѓжаЕФЮяжЪ |
|
A | CuКЭЯЁЯѕЫс | NO | NaOHШмвК | |
B | ХЈбЮЫсКЭ |
| NaOHШмвК | |
C | бЧСђЫсФЦКЭХЈСђЫс |
| ЦЗКьШмвК | |
D | ХЈАБЫЎКЭCaO |
| ЗгЬЊШмвК |
A.AB.BC.CD.D
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЛЏбЇбЇЯАаЁзщгћЬНОПМКЭщЕФаджЪЃЌгУМКЭщНјааЯТСаЪЕбщЃК
ЪЕбщ1ЃК

ЪЕбщ2ЃКНЋЩЯЪіГШЩЋШмвКзАШыУмЗтадКУЕФЮоЩЋЪдМСЦПжаЁЃЙ§вЛЖЮЪБМфЃЌШмвКбеЩЋБфЧГЃЌДђПЊЦПИЧЦППкГіЯжАзЮэЁЃ
ЪЕбщ3ЃКРћгУШчЭМЫљЪОЪЕбщзАжУдквЛЖЈЬѕМўЗжНтМКЭщЃЈЩњГЩБћЭщКЭБћЯЉ![]() ЃЉЃЌЧвБћЯЉФмБЛЫсад
ЃЉЃЌЧвБћЯЉФмБЛЫсад![]() ШмвКбѕЛЏЁЃ
ШмвКбѕЛЏЁЃ
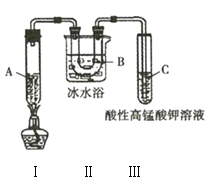
(1)ЪЕбщ1жагУЕНЕФВЃСЇвЧЦїжївЊга______________________________________ЃЈЬюУћГЦЃЉЁЃ
(2)гЩЪЕбщ1ПЩжЊЃЌМКЭщЕФЮяРэаджЪга____________________________________ЁЃ
(3)ЪЕбщ2жаЕФГШЩЋШмвКж№НЅБфЧГЕФдвђЪЧ__________________ЃЈЬюађКХЃЉЁЃ
A.МКЭщгыфхЗЂЩњСЫШЁДњЗДгІ
B.фхДњМКЭщЮЊЮоЩЋЮяжЪ
C.вКфхЯђЭтЛгЗЂХЈЖШНЕЕЭ
D.МКЭщгывКфхЗЂЩњСЫМгГЩЗДгІ
E.вКфхгыМКЭщЗжВуЃЌУмЖШДѓЕФвКфхдкЯТВу
(4)ЪЕбщ3жазАжУЂђЕФзїгУЪЧ______________________________________ЁЃ
(5)ЪдЙмCжаЕФЯжЯѓЪЧ______________________________________ЁЃ
(6)ЪдаДГізАжУЂёжаЗЂЩњЕФЩњГЩБћЭщКЭБћЯЉЕФЛЏбЇЗНГЬЪНЃК______________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЭЕФЛЏКЯЮяШчCu2OЁЂCuOЁЂCuClЁЂCuSO4ЁЄ5H2OЁЂCu(IO3)2ЁЂ[Cu(H2NCH2CH2NH2)2]Cl2ЕШОљгазХЙуЗКЕФгІгУ.ЛиД№ЯТСаЮЪЬтЃК
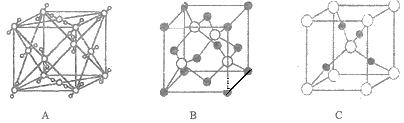
(1)ЛљЬЌCu2+ЕФКЫЭтЕчзгХХВМЪНЮЊ____________
(2)IO3-ЕФПеМфЙЙаЭЮЊ_____________________________ЃЈгУЮФзжУшЪіЃЉ,гыSO42-ЛЅЮЊЕШЕчзгЬхЕФЗжзгЮЊ____________
(3)ХфРызг[Cu(H2NCH2CH2NH2)2]2+жаЃЌCu2+ЕФХфЮЛЪ§ЪЧ____________.
ЂйввЖўАЗЗжзгжаN дзгЙьЕРЕФдгЛЏРраЭЮЊ____________.
Ђк 1mol [Cu(H2NCH2CH2NH2)2]2+ жаКЌгаЕФІвМќЕФЪ§ФПЮЊ____________.
(4)CuOЕФШлЕуБШCuClЕФШлЕуИп,ЦфдвђЪЧ____________.
(5)Cu2OОЇЬхНсЙЙПЩФмЪЧ____________ (ЬюзжФИ).
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com