
ʵ����Ҫ0.1 mol/L NaOH��Һ450 mL��������Һ�����е�����ش��������⣺
��1��ʵ���г���������ƽ���ձ�������ƿ���Ҫ������������___ ___
_______________________________________________ __________��
��2�����ݼ����֪������NaOH������Ϊ________g��
��3������һ�����ʵ���Ũ����Һ��ʵ���У�����������²���
| A������ʱ������������� |
| B����NaOH����ֽ���ϳ��� |
| C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�� |
| D��������ƿת��ʱ��������Һ�彦�� |
��1������������ͷ�ιܡ�ҩ�ȡ���2��2����3��AC
���������������1��ȡNaOH��Ҫ��ҩ�ף������������Ҫ�ò�������������Ҫ�ý�ͷ�ιܡ�
��2��ʵ����Ҫ0.1 mol/L NaOH��Һ450 mL����������ƿ�Ĺ����Ҫ����NaOH��Һ500mL������NaOH������Ϊ��0.5L��0.1mol/L��40g/mol=2g��
��3��������Һ��Ũ��ƫ��ƫ����Ҫ�������ƹ����������Ƿ���ʧ����Һ������ܵ���Ӱ�졣A����������������C��С����Һ��������ߵ���������Һ��Ũ��ƫ�ߣ�B��D��E��ʧ�����ʣ�F��H��������Һ�����������Һ��Ũ��ƫ�͡�
���㣺���⿼������һ�����ʵ���Ũ����Һ���������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ӵ�ʳ���к��еĵ������һ�ְ�ɫ�ᾧ��ĩ�������º��ȶ���������560�濪ʼ�ֽ⡣�����������µ������һ�ֽ�ǿ��������������⻯��������εȻ�ԭ�����ʷ�Ӧ����ҵ��������ص��������£�
��1����������������ص���Ҫ������ ��
��2�������±�����ص��ܽ�ȣ������۵õ�����ؾ��壬�ɾ��� �����ˡ�ϴ�ӡ�����Ȳ��衣
| �¶�/�� | 20 | 40 | 60 | 80 |
| KIO3/100gˮ | 8.08 | 12.6 | 18.3 | 24.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʵ��������ܴﵽԤ��Ŀ�ĵ���( )
������AlCl3��Һ�ɵô�������ˮ�Ȼ�������ֽ���������������Ӻ�ͭ����ʵ���У����������ֽ�����ɺ��ܽ���ֽ�¶˽���չ�����н���ʵ�飻���ù㷺pH��ֽ���ij��ҺpHΪ3.5���ܵ����̪��Һȷ�������������ռ��������ֱ�Ӽ���ʳ�������Ƿ��е�Ԫ�أ�������ʽ�ζ�����ȡ20.00 mL�������������Һ������������Ȼ�̼��Һ��ȥ���ڼ����е���ϩ����
| A���ޢ� | B���ڢ� | C���ݢޢ� | D��ֻ�Т� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ͭ�����ڲ�ͬ�¶��¿�ʧȥ���ֻ�ȫ���ᾧˮ�����ֽ�������ijѧ���ڲ�ͬ�¶��¸�8.000 g����ͭ������ȣ��¶������ߣ���ʵ������¼���£�
| ʵ����� | �¶ȣ��棩 | ��ȴ��ʣ������������g�� |
| 1 | 102 | 6.848 |
| 2 | 113 | 5.696 |
| 3 | 258 | 5.120 |
| 4 | 570 | 2.560 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������(FeSO4��7H2O)��ҽҩ������Ѫ����Ϊ�ⶨ��Ѫ������Ԫ�صĺ�����ij��ѧ��ȤС�����������ʵ�鷽����
����һ���ζ�����������KMnO4��Һ�ζ��ⶨ��Ԫ�صĺ�����
��Ӧԭ����5Fe2����MnO4����8H��===5Fe3����Mn2����4H2O
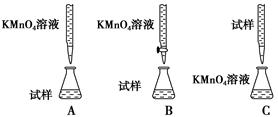
(1)ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����________(����������)��
(2)����ʵ����KMnO4��Һ��Ҫ�ữ�������ữ������________��
| A��ϡ���� | B��Ũ���� | C��ϡ���� | D��ϡ���� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ȼ��Ƴ����ڵ�·�ڱ��������������������ˮ�������������ʡ�ʵ�����ù�ҵ����ʯ����������Al2O3��Fe2O3�����ʣ��Ʊ��Ȼ��Ƶ���Ҫ�������£� ���������գ�
���������գ�
��1������ʹ�õ���������ʵ���Ũ��ԼΪ6.0mol/L������36.5%�����ᣨ�ܶ�Ϊ1.2g/mL������6.0mol/L������100mL������IJ��������в���������Ͳ����ͷ�ιܡ� ����Ҫ��ȡ36.5%������ mL�����ƹ����У���������������ȷ�����в���������Ũ��ƫС���� ��
| A������ҡ�Ⱥ���Һ����ڿ̶��� |
| B������ʱ��������ƿ�Ŀ̶��� |
| C������Һת������ƿ��û��ϴ���ձ��Ͳ���������ת�붨�ݲ��� |
| D�������ˮ�����˿̶��ߣ�ȡ������ˮʹҺ��ǡ�õ��̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij������ˮ�к�����̬��(�������ȷ���Cl2)��ͨ������ʵ��ⶨ��Ũ�ȣ�
��ȡ��ˮ��10.0mL����ƿ������10.0 mL KI��Һ(����)������ָʾ��2��3�Ρ�
��ȡһ�ζ�������������ˮ������ˮϴ����Ȼ���ע��0.01 mol��L��1 ��Na2S2O3��Һ������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2 + 2Na2S2O3 =" 2NaI" + Na2S4O6 (Na2S4O6��ҺΪ��ɫ)���Իش��������⣺
��1������ټ����ָʾ���� ��
��2������ٷ�Ӧ�����ӷ���ʽ�� ��
��3�����ۢ۵�����Һ�� ɫ��Ϊ ɫ��30s���ٱ仯�����յ㣬����ȥNa2S2O3��Һ20.00mL�����ˮ��C12�����ʵ���Ũ��Ϊ ��
��4����������ʵ����������ᵼ������õ�Cl2�����ʵ���Ũ�Ȼ��ʵ��Ũ�� (�ƫ����ƫС������ȡ�)��
��5������Na2S2O3��Һ��������淶��û��ƽ�ӣ��ζ�ǰ���ӣ��ζ����ָ��ӣ����ᵼ������õ�Cl2�����ʵ���Ũ�Ȼ��ʵ��Ũ�� (�ƫ����ƫС������ȡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��.��1��ij��ѧ��ȤС����������װ����ѡȡ��Ҫ��װ����ȡ��NH4��2SO4��Һ�����ӵ�˳���ýӿ������ĸ��ʾ���ǣ�a ��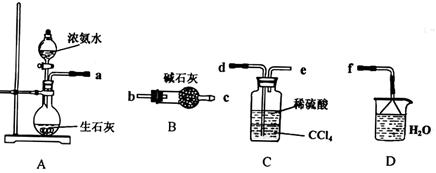
��2����װ��C������Һ����뿪�IJ���������_________��װ��D�������� ��
��.�������ƿ������ڸ��Ƶر�ˮ�ʡ��������ؽ������ӷ�ˮ�������ೱ��Ҳ������Ӧ�������ȡ���ҵ�������������Ƶ���Ҫ�������£�
��֪CaO2��8H2O�ʰ�ɫ������ˮ��I2+2S2O32��= 2I��+S4O62��
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ�� ��
��2�����顰ˮϴ���Ƿ�ϸ�ķ����� ��
��3���ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g��Ʒ����ƿ�У�������������ˮ������b g KI���壬�ٵ�������2 mol/L��H2SO4��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol��L��1��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺV mL��
���жϴ˵ζ�ʵ��ﵽ�յ�ķ����ǣ� ��
��CaO2����������Ϊ (����ĸ��ʾ)��
��ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�CaO2��������������
�������Ӱ�족����ƫ�͡���ƫ�ߡ�����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ظ����ƣ�Na2Cr2O7?2H2O���׳ƺ췯�ƣ��ڹ�ҵ�����й㷺��;���ҹ�Ŀǰ��Ҫ���Ը�������Ҫ�ɷ�ΪFeO?Cr2O3��������Al2O3��MgO��SiO2�����ʣ�Ϊ��Ҫԭ�Ͻ�������������Ҫ�����������£�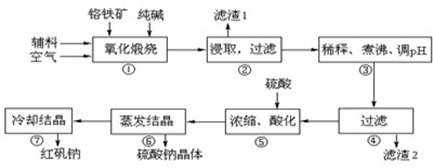
���漰����Ҫ��Ӧ�У�
����Ӧ��4FeO?Cr2O3+8Na2CO3+7O2  8Na2CrO4+2Fe2O3+8CO2
8Na2CrO4+2Fe2O3+8CO2
����Ӧ�� SiO2+Na2CO3 Na2SiO3+CO2�� Al2O3+Na2CO3
Na2SiO3+CO2�� Al2O3+Na2CO3 2NaAlO2+CO2��
2NaAlO2+CO2��
����������������������ʽ��ȫ����ʱ��Һ��pH��
| ������ | Al(OH)3 | Fe(OH)3 | Mg(OH)2 | Cr(OH)3 |
| ��ȫ����ʱ��ҺpH | 4.7 | 3.7 | 11.2 | 5.6 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com