

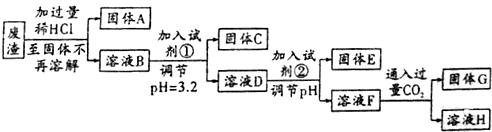
���� �����̿�֪��������������������̷�Ӧ���������̣���MnCO3������ҺpH��ʹ��Һ��Al3+��Fe3+ת��Ϊ���������˳�ȥ��MnS��ͭ�������ӻ�ԭΪ���ʣ����˷��룬��Һ�м����������������̷�Ӧ���ɶ������̣�ͨ�����˻�ö������̣�
��1����������ʵ���˷�������ۺ����á�����ļ��٣�
��2����������������̷�Ӧ���ɶ������̣�����������أ���Ԫ���غ��֪���������
��3����MnCO3������Һ�е��ᣬʹAl3+��Fe3+ˮ�������������������MnS����Һ�е�Cu2+��Ӧ����CuS������Mn2+��aq�����dz���ת����
��4��MnO2�����������������ϣ������������Һ�Ʊ�MnO2��MnԪ�ط���������Ӧ����װ��ͼ��֪��B�缫�����������ɣ�������ԭ��Ӧ��Ӧ�������ӷŵ�����������AΪ��������������Mn2+ʧȥ���ӵõ�MnO2���ɵ���غ��֪����H+���ɣ�������ͨ�����ӽ���Ĥ�������ң����ݵ���ת�ƿ�֪�������ҵ��������������������ҵ������Һ�������䣬������Һ�������仯��Ϊ�����������������������ӵ�������
��5�������յ�SO2ת��ΪMnSO4���������ʵ�����ȣ���ȥ��������ͭ����������ʱ̼����Ҳת��ΪMnSO4�����з�����Ӧ��2KMnO4+3MnSO4+2H2O=5MnO2��+K2SO4+2 H2SO4���ɵù�ϵʽ��3SO2��3MnSO4��5MnO2���ɹ�ϵʽ����n��MnSO4��������MnԪ���غ���������MnԪ�����ʵ�����������MnԪ�����ʵ������൱������Ķ������̵����ʵ�����ȣ�
��� �⣺�����̿�֪��������������������̷�Ӧ���������̣���MnCO3������ҺpH��ʹ��Һ��Al3+��Fe3+ת��Ϊ���������˳�ȥ��MnS��ͭ�������ӻ�ԭΪ���ʣ����˷��룬��Һ�м����������������̷�Ӧ���ɶ������̣�ͨ�����˻�ö������̣�
��1����������ʵ���˷�������ۺ����á�����ļ��٣����ɫ��Ⱦ�أ�
�ʴ�Ϊ��AC��
��2����������������̷�Ӧ���ɶ������̣�����������أ���Ԫ���غ��֪���������ᣬ��Ӧ����ʽΪ��2KMnO4+3MnSO4+2H2O=5MnO2��+K2SO4+2 H2SO4��
�ʴ�Ϊ��2KMnO4+3MnSO4+2H2O=5MnO2��+K2SO4+2 H2SO4��
��3����MnCO3�ܳ�ȥ��Һ��Al3+��Fe3+����ԭ���ǣ�������Һ�е��ᣬ�ٽ�Al3+��Fe3+ˮ���������������������MnS��ȥ��Һ�е�Cu2+�����ӷ���ʽΪ��MnS��s��+Cu2+��aq��=CuS��s��+Mn2+��aq����
�ʴ�Ϊ��������Һ�е��ᣬ�ٽ�Al3+��Fe3+ˮ�������������������MnS��s��+Cu2+��aq��=CuS��s��+Mn2+��aq����
��4��MnO2�����������������ϣ������������Һ�Ʊ�MnO2��MnԪ�ط���������Ӧ����װ��ͼ��֪��B�缫�����������ɣ�������ԭ��Ӧ��Ӧ�������ӷŵ�����������AΪ��������������Mn2+ʧȥ���ӵõ�MnO2���ɵ���غ��֪����H+���ɣ�������ͨ�����ӽ���Ĥ�������ң������缫��ӦʽΪ��Mn2++2H2O-2e-=MnO2+4H+�����ݵ���ת�ƿ�֪�������ҵ��������������������ҵ������Һ�������䣬������Һ�������仯��Ϊ�����������������������ӵ����������Ʊ�1mol MnO2��ת�Ƶ���Ϊ2mol�����������ҵ�������Ϊ2mol����Ĥ������Һ�������仯���m��-��m����Ϊ1mol��87g/mol+2mol��1g/mol=89g��
�ʴ�Ϊ��Mn2++2H2O-2e-=MnO2+4H+��89��
��5�������յ�SO2ת��ΪMnSO4���������ʵ�����ȣ���ȥ��������ͭ����������ʱ̼����Ҳת��ΪMnSO4�����з�����Ӧ��2KMnO4+3MnSO4+2H2O=5MnO2��+K2SO4+2 H2SO4���ɵù�ϵʽ��3SO2��3MnSO4��5MnO2���ɹ�ϵʽ��֪n��MnSO4��=$\frac{3}{5}$��$\frac{1000cg}{87g/mol}$���������MnԪ��Ϊ$\frac{3}{5}$��$\frac{1000cg}{87g/mol}$-$\frac{1000aL}{22.4L/mol}$��b%��67.2%����MnԪ���غ��֪�൱������Ķ����������ʵ���Ϊ��$\frac{3}{5}$��$\frac{1000cg}{87g/mol}$-$\frac{1000aL}{22.4L/mol}$��b%��67.2%������������Ϊ��$\frac{3}{5}$��$\frac{1000cg}{87g/mol}$-$\frac{1000aL}{22.4L/mol}$��b%��67.2%����87g/mol=��600c-26.1ab��g����������Ϊ��0.6c-0.0261ab�� Kg��
�ʴ�Ϊ����0.6c-0.0261ab����
���� ���⿼�������Ʊ��������̣��ؼ��ǶԹ�������ͼԭ�������⣬��4���м���Ϊ�״��㣬ѧ�������Ժ���������������ͨ�����ӽ���Ĥ������������5����ע�����MnԪ���غ㼰��ϵʽ���㣬��Ŀ�ѶȽϴ�


 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ��Ũ�Ⱦ�Ϊ0.1mol/L NaCl��KI���Һ����μ���AgNO3��Һ | �ȳ��ֻ�ɫ���� | �ܽ��ԣ�AgCl��AgI |
| B | ��������NaOH���Ҵ���Һ���ȣ����ݳ�����ͨ�����Ը��������Һ�� | ��Һ��ɫ��ȥ | ֤������ϩ���� |
| C | ��X����Һ�е���ŨNaOH��Һ���������ɫʯ����ֽ���ڹܿ� | ���������� | X��Һ����NH4+ |
| D | ��SO2����ͨ��Ba��NO3��2��Һ | ������ɫ���� | ����ΪBaSO3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | X��Y������λ��ͬһ���� | |
| B�� | X��Y�������γ�ԭ�Ӹ�����Ϊ1��1�����ӻ����� | |
| C�� | X������Y���ӵĵ��Ӳ�ṹ������ͬ | |
| D�� | X��Y�γɵĹ��ۻ������У���ԭ�������һ������8���ӵ��ȶ��ṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ƾ��� | B�� | ������������ | C�� | Ũ���� | D�� | Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ������������ʽ��ȫ����ʱ����pH | �������������ȫ�ܽ�ʱ����pH |
| Fe3+ | 3.2 | |
| A13+ | 5.3 | 11.9 |
| Mg2+ | 12.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ۢ� | B�� | �ۢܢ� | C�� | �ܢ� | D�� | �ܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��ϩ��3-��-1-��ϩ | B�� | ������Ȳ | ||
| C�� | 2-��-3-�һ�-1-��ϩ�ͻ����� | D�� | ��֬���Ӳ֬�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ס������Թܶ��а�ɫ�������� | |
| B�� | �ס������Թܶ�û�а�ɫ�������� | |
| C�� | ���Թ�û�а�ɫ�������ɶ����Թ��� | |
| D�� | ���Թ��а�ɫ�������ɶ����Թ�û�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com