
����Ŀ����1��25��ʱ��Ũ��Ϊ0.1 mol��L��1��6����Һ����HCl�� ��CH3OOH�� ��Ba(OH)2����Na2CO3����KCl����NH4Cl��ҺpH��С�����˳��Ϊ__________________(��д���)��
��2��25��ʱ������ĵ��볣��Ka=1.7��10-5mol/L������¶���CH3COONa��ˮ��ƽ�ⳣ��Kh=_________mol ��L-1��������С�����һλ����
��3��25��ʱ��pH��3�Ĵ����pH��11������������Һ�������Ϻ���Һ��_________���������������������������������� ����д����Һ������Ũ�ȼ��һ����ʽ��________________________________��
��4��25��ʱ����m mol/L�Ĵ����n mol/L������������Һ�������Ϻ���Һ��pH��7������Һ��c(CH3COO��) + c(CH3COOH)=______��m��n�Ĵ�С��ϵ�ǣ�_____����� ��������������<������
��5��25��ʱ����������������ʵ���Ũ�ȵĴ����백ˮ��Ϻ���Һ��pH��7 ����NH3��H2O�ĵ��볣��Ka=______________��
���𰸡��٢ڢޢݢܢ� 5.9��10-10 ���� c(Na��) + c(H��) = c(CH3COO��) + c(OH��) ![]() �� .7��10-5mol/L
�� .7��10-5mol/L
��������
��1����HCl��һԪǿ�ᣬ ��CH3OOH��һԪ���ᣬ ��Ba(OH)2�Ƕ�Ԫǿ���Na2CO3��ǿ�������Σ���KCl��ǿ��ǿ���Σ���NH4Cl��ǿ�������Ρ����ԣ�ǿ������������ǿ�������Σ����ԣ���Ĵ���ǿ�������εġ������⼸����ҺpH��С�����˳��Ϊ�٢ڢޢݢܢ�.
��2��KHAc![]() CH3COO-+H+,
CH3COO-+H+,![]() �����¶���CH3COONa��ˮ��ƽ��ΪCH3COO-+H2O
�����¶���CH3COONa��ˮ��ƽ��ΪCH3COO-+H2O![]() CH3COOH+OH-��ˮ��ƽ�ⳣ��
CH3COOH+OH-��ˮ��ƽ�ⳣ��![]()
![]()
![]() ������
������![]() ��
��
��3��25��ʱ��pH��3�Ĵ��ᣬc(H+)=10-3mol/L�� pH��11������������Һ,c(H+)=10-11mol/L����c(OH-)=Kw��c(H+)=10-14��10-11=10-3mol/L��������Һ�е�����Ũ����ȡ����������Ϻ���IJ���ǡ����ȫ�к͡������ڴ���Ϊ���ᣬ���д���δ����Ĵ�����Ӵ��ڣ�������������H+��CH3COO-��������Һ�����ԡ�����Һ�д��ڵ���غ㡣c(Na��) + c(H��) = c(CH3COO��) + c(OH��)��
��4��������ҺΪ�������ϣ�������Һ��c(CH3COO��) + c(CH3COOH)=![]() mol/L����Ϊ�������ᣬ����ǿ��������ʵ�����ϣ�ǡ������CH3COONa����Һ����CH3COO-��ˮ���Լ��ԡ�Ϊ��ʹ��Һ�����ԣ������������һЩ�����������������ˮ��ļ��ԡ�����m��n�Ĵ�С��ϵ��m��n��
mol/L����Ϊ�������ᣬ����ǿ��������ʵ�����ϣ�ǡ������CH3COONa����Һ����CH3COO-��ˮ���Լ��ԡ�Ϊ��ʹ��Һ�����ԣ������������һЩ�����������������ˮ��ļ��ԡ�����m��n�Ĵ�С��ϵ��m��n��
��5��25��ʱ����������������ʵ���Ũ�ȵĴ����백ˮ��Ϻ���Һ��pH��7 ��˵��������һˮ�ϰ���ǿ���̶���ͬ��Ҳ���ǵ���̶���ȡ����ڴ���ĵ���ƽ�ⳣ��ΪKa=1.7��10-5mol/L������NH3��H2O�ĵ��볣��Ka=1.7��10-5mol/L��


 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���ҩ��ҵ�г������·����ϳ�һ��ҩ���м���(G)��


��1��G�ķ���ʽ��___��
��2��������A�к��������ŵ�����Ϊ___��
��3����C��D�ķ�Ӧ����Ϊ___��������E�Ľṹ��ʽΪ___��
��4��д��B��C�ķ�Ӧ�Ļ�ѧ����ʽ��___��
��5��д��ͬʱ��������������B��һ��ͬ���칹��Ľṹ��ʽ��___��
����������Cu(OH)2�ڼ��������·�Ӧ����ש��ɫ������ˮ�����֮һ����FeCl3��Һ������ɫ��Ӧ��
�ں˴Ź�������Ϊ����壬�������Ϊ1��2��4��9��
�۷����к��а�����
��6����֪��RCN![]() RCH2NH2����д����HOOCCH2CH2COOH��CH3CH2ClΪԭ���Ʊ�
RCH2NH2����д����HOOCCH2CH2COOH��CH3CH2ClΪԭ���Ʊ�![]() �ĺϳ�·������ͼ___(���Լ�����)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ___(���Լ�����)���ϳ�·������ͼʾ�����£�
CH3CH2OH![]() CH2
CH2![]() CH2
CH2![]() CH3CH2Cl
CH3CH2Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ����( )
A.��Ӧ5NH4NO3��2HNO3��4N2����9H2O������22.4LN2ʱת�Ƶĵ�����Ϊ3.75NA
B.n(H2SO3)��n(HSO3��)��1mol��NaHSO3��Һ�У�����Na������Ŀ����NA
C.��״���¼���������Ļ�����干22.4L����ȫȼ�պ����ķ�������һ��ΪNA
D.10g��D2O�к��е����������������ֱ�Ϊ5NA��4NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л���ĽṹΪ��ͼ��ʾ�����������л�������ȷ��˵����
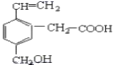
�ٸ����ʷ���ʽΪC11H12O3��������ˮ��
����ʹ��ˮ������KMnO4��Һ��ɫ����ԭ����ͬ��
�۷ֱ���Na��NaHCO3��Ӧ����������������ʵ���֮�Ⱦ���1:1��
���ܷ���ȡ�����ӳɡ�ˮ�⡢��������ԭ��Ӧ
A.1��B.2��C.3��D.4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���йؼס��ҡ��������ĸ�ͼʾ��������ȷ���ǣ� ��

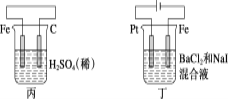
A.���и�����ӦʽΪ2H����2e��=H2��
B.����������ӦʽΪAg����e��=Ag
C.����H����̼�������ƶ�
D.���е�ʼʱ������������ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO��SO2�Ǵ�����Ⱦ���壬���û�ѧ��Ӧ��������Ⱦ����Ҫ������
��.�״����Բ���Ͳ������ʯ��ȼ�ϣ�������Դ���ţ�����CO���Ժϳɼ״���CO+2H2![]() CH3OH(g)��һ�������£����ݻ�ΪVL���ܱ������г���a mol CO��2a mol H2�ϳɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g)��һ�������£����ݻ�ΪVL���ܱ������г���a mol CO��2a mol H2�ϳɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

(1)������˵����Ӧ�ﵽƽ��״̬����_______(�����)��
��v��(CO)=2v��(H2)
��c(CO)=c(CH3OH)
�ۻ�������ƽ����Է�����������
�ܵ�λʱ��������2n mol H2��ͬʱ����n mol CH3OH
(2)�÷�Ӧ��A���ƽ�ⳣ��K_________(��a��V��ʾ)��
(3)д����������v(CO)�������COת���ʵ�һ���ʩ��________
��.ijѧϰС����SO2Ϊԭ�ϣ����õ绯ѧ������ȡ���ᡣ
(4)ԭ���ԭ������С����Ƶ�ԭ��ʾ��ͼ(��ͼ1��ʾ)��д���õ�������ĵ缫��Ӧʽ_______��

(5)���ԭ������С����Na2SO3��Һ�������SO2�õ�NaHSO3��Һ��Ȼ�������Һ�Ƶ�������(ԭ����ͼ2��ʾ)��д����ʼ���ʱ�����ĵ缫��Ӧʽ_______��
��.���������(Na2S2O3)�׳ƴ��մ����Ź㷺����;����SO2����Na2S2O3��ijС��ͬѧ�Ʊ���Ԥ�Ⲣ̽����������Ƶ�����(��Ӧ������Һ�н���)��
Ԥ�� | ʵ����� | ʵ������ | |
̽��1 | Na2S2O3��Һ�ʼ��� | ��pH��ֽ���ڲ���Ƭ�ϣ��ò�����պȡ��Һ������ֽ�� | pH=8 |
̽��2 | Na2S2O3���л�ԭ�� | ��������ˮ�еμ�Na2S2O3��Һ | ����ɫ��ɫ��dz��������ȥ |
(6)����SO2���Ʊ�Na2S2O3������������_________��
(7)�����ӷ���ʽ��ʾNa2S2O3��Һ���м��Ե�ԭ��_________��
(8)̽��2��Ӧ�����ӷ���ʽΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��25�棬101kPa�£�lg C8H18�����飩����Է���������114��ȼ�����ɶ�����̼��Һ̬ˮʱ�ų�48.40kJ��������ʾ������Ӧ���Ȼ�ѧ����ʽ��ȷ���ǣ� ��
A. C8H18��1����25/2O2��g����8CO2��g����9H2O��g������H����48.40kJ��mol��1
B. C8H18��1����25/2O2��g����8CO2��g����9H2O��1������H����5518kJ��mol��1
C. C8H18��1����25/2O2��g����8CO2��g����9H2O��1������H����5518kJ��mol��1
D. C8H18��1����25/2O2��g����8CO2��g����9H2O��1������H����48.40kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Դ�����ķ�չ,����Դ��ؼ���Ҳ�ڲ��ϴ���,���͵�����ӵ��һ����LiCoO2��LiFePO4��Ϊ�������ϣ���ʯī̼Ϊ�������ϣ�������LiPF6�ȵ��л���ҺΪ�������Һ��
��1��Pԭ�ӵĵ����Ų�ʽΪ_________��Fe2+��δ�ɶԵ�����Ϊ___________��
��2��N��O��Fԭ�ӵĵ�һ��������С�����˳��Ϊ_______��
��3���ȵ�����������ƵĻ�ѧ�����������ǵ���������������ġ�ClO4-��PO43-��Ϊ�ȵ����壬ClO4-�����幹��Ϊ_______������ԭ�ӵ��ӻ��������Ϊ________��
��4������ͬϵ���У�CH4�ķе���ͣ�ԭ����______________��
��5����CuSO4��Һ�м��백ˮ�������γ���ɫ�����������Ӱ�ˮ�������ܽ⣬�õ�����ɫ��Һ���ڴ���Һ�м����Ҵ�����������ɫ�ľ��塣����ɫ�����õ�����ɫ��Һ�����ӷ���ʽΪ_______________������ɫ�����д��ڵĻ�ѧ��������__________ ��������ţ�
A�����Ӽ�
B������
C���Ǽ��Թ��ۼ�
D�����
E��������
F�����
��6����ͼ��ʾΪCo��ij��������ľ����ṹͼ�����������Ļ�ѧʽΪ______�����þ������ⳤΪa pm,��þ�����ܶ�Ϊ_____________g/cm3����NAΪ�����ӵ�������ֵ��
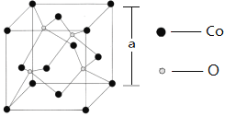
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��﮵�ijЩ����������������IJ��ϡ���ش�
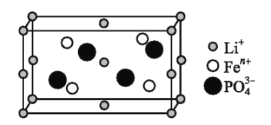
��1����ͼ��ij�綯��������������ϵľ����ṹʾ��ͼ���仯ѧʽΪ ______ ������![]() �Ļ�̬�����Ų�ʽΪ ______ ��
�Ļ�̬�����Ų�ʽΪ ______ ��![]() �Ŀռ乹��Ϊ ______ ��
�Ŀռ乹��Ϊ ______ ��
��2��![]() ��Na�е�һ�����ܽ�С��Ԫ���� ______ ��LiF��NaCl�������۵�ϸߵ��� ______ ��
��Na�е�һ�����ܽ�С��Ԫ���� ______ ��LiF��NaCl�������۵�ϸߵ��� ______ ��
��3���������һ�����õĴ�����ϣ����������м���ʱ�����������õ������![]() ���⻯ﮣ��⻯﮵ĵ���ʽΪ ______ ��������Ӧ�Ļ�ѧ����ʽΪ ______��
���⻯ﮣ��⻯﮵ĵ���ʽΪ ______ ��������Ӧ�Ļ�ѧ����ʽΪ ______��
��4�������Ϊ������������������λ��Ϊ ______ �����侧���߳�Ϊapm����﮾�����ԭ�ӵĿռ�ռ������ ______ ��
��5���л���Լ����л��ϳ�������ҪӦ�ã���������![]() ��
��![]() �ȷ�Ӧ������˵������ȷ���� ______
�ȷ�Ӧ������˵������ȷ���� ______ ![]() ����ĸ���
����ĸ���![]() ��
��
A.CO2����������������Ŀ��Ϊ1��1
B.����̬�ͻ���̬�Ԫ�ؾ�����������������
C.�嶡���(C4H9Li)��̼ԭ�ӵ��ӻ��������Ϊsp3��sp2��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com