
����Ŀ���绯ѧԭ���ڷ�ֹ������ʴ������ת�������ʺϳɵȷ���Ӧ�ù㷺��

��1��ͼ1�У�Ϊ�˼�����ˮ�Ը�բ��A�ĸ�ʴ������B����ѡ��__________������ĸ��ţ�
a��̼�� b��п�� c��ͭ��
�õ绯ѧԭ�����Ͳ���B�趨�ڲ�ԭ��_______________��

��2��þȼ�ϵ���ڿ��ƶ������豸��Դ�ͱ��õ�Դ�ȷ���Ӧ��ǰ��������ͼ2Ϊ��þ������������ȼ�ϵ��ԭ��ʾ��ͼ���缫Ϊþ�Ͻ�Ͳ��Ͻ�
��EΪ��ȼ�ϵ�ص�_____________������������������������F�缫�ϵĵ缫��ӦʽΪ_____________��
��þȼ�ϵ�ظ����������Ը�ʴ����������ʹ���������ʽ��ͣ��û�ѧ���������ԭ��____________��
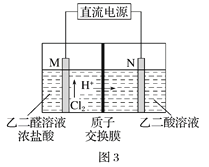
��3����ȩ�ᣨHOOC-CHO�����л��ϳɵ���Ҫ�м��塣��ҵ������˫���ҳɶԵ�ⷨ��������ȩ�ᣬԭ����ͼ3��ʾ����װ��������������Ϊ���Ե缫�������Ҿ��ɲ�����ȩ�ᣬ�����Ҷ�ȩ��M�缫�IJ��ﷴӦ������ȩ�ᡣ
��N�缫�ϵĵ缫��ӦʽΪ______________��
������2molH+ͨ�����ӽ���Ĥ������ȫ�����˷�Ӧ�����װ�������ɵ���ȩ��Ϊ________________mol��
���𰸡���1��b��п����ԭ��صĸ�������Zn -2e- = Zn2+�����������ܸ�ʴ���趨�ڲ�
��2���� ����ClO��+2e��+H2O = Cl��+2OH����
�� Mg+2H2O= Mg(OH)2+H2����
��3���� HOOC-COOH+2e��+2H+ ="HOOC-CHO+" H2O���� 2��
�������������������1���γ�ԭ���ʱ��Fe����������������Ҫѡ������Ա�Feǿ�Ľ���������������ѡп��п�Ļ����Ա�Feǿ�����������������ܸ�ʴ���趨�ڲ��ʴ�Ϊ��b��п����ԭ��صĸ�������ʧ���ӣ�Zn-2e-�TZn2+�����������ܸ�ʴ���趨�ڲ�
��2���١�þ-����������ȼ�ϵ����ʧ���ӵ�Ϊ��������MgΪ������������ClO-�õ������������ӣ��������ĵ缫��ӦʽΪ��ClO-+2e-+H2O�TCl-+2OH-���ʴ�Ϊ������ClO-+2e-+H2O�TCl-+2OH-��
��Mg�Ļ����Խ�ǿ����ˮ��Ӧ�����������䷴ӦΪ��Mg+2H2O�TMg��OH��2+H2�����ʴ�Ϊ��Mg+2H2O�TMg��OH��2+H2����
��3����N�缫��HOOC-COOH�õ�������HOOC-CHO����缫��ӦʽΪHOOC-COOH+2e-+2H+ �THOOC-CHO+H2O���ʴ�Ϊ��HOOC-COOH+2e-+2H+�THOOC-CHO+H2O��
��2mol H+ͨ�����ӽ���Ĥ��������ת��2mol���ӣ����ݵ缫����ʽHOOC-COOH+2e-+2H+ �THOOC-CHO+H2O����֪����1mol��ȩ�ᣬ��������������ȩ�������������ɵ���ȩ��Ϊ2mol���ʴ�Ϊ��2��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС��̽��SO2���廹ԭFe3����I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ��
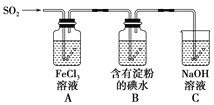
(1)Aװ���з�����Ӧ�����ӷ���ʽΪ_________________________________________��
(2)Bװ���з�����Ӧ�����ӷ���ʽΪ_________________________________________��
(3)����ʵ�鷽����������ʵ������ȡ����SO2����________��
A��Na2SO3��Һ��HNO3 B��Na2SO3������Ũ����
C���������ڴ�����ȼ�� D��ͭ����ŨH2SO4
(4)װ��C��������__________________________________________________��
(5)��Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ���������________(�����)��
A��������B��ʯ��������C��©������D���ձ� E������������F������
(6)������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3��������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м�������KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ�м���KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ�����������Һ�м�����ϡ�����ữ��BaCl2��������ɫ������
������������������________��ԭ����____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б�״����V L ����AB3������Ħ������ΪM g��mol-1����
��1������������ԭ������Ϊ________________��(�г�����ʽ����,��ͬ)��
��2�������������Ϊ_____________g��
��3������������1Lˮ�У������Ƿ�Ӧ��������Һ�����ʵ���������Ϊ______________��
��4������������ˮ���γ�1 L��Һ������Һ�����ʵ���Ũ��Ϊ_______________ mol��L-1��
��5������������1Lˮ�У������Ƿ�Ӧ������������Һ���ܶ�Ϊ��g��mL-1������Һ�����ʵ���Ũ��Ϊ_____________________mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������
��1����֪�����Ļ�ѧʽ��H2����Ħ������Ϊ______��4 g H2�����ʵ���Ϊ__mol�����״���µ����Ϊ_____L���京H2���ӵĸ���Ϊ________��������ԭ�ӵĸ���Ϊ__________��
��2��ijѧ������12 mol��L��1Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.60 mol/L��ϡ���ᡣ��ѧ����Ҫ��ȡ___________ mL����Ũ����������ơ�
��3������21.6g��CO��CO2��ɵĻ�����壬�ڱ�״���������Ϊ13.44L���ش��������⣺�û�������ƽ��Ħ������Ϊ_____________���û�������к�CO2�����ʵ���Ϊ__mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڶԵ����˶�״̬�������У�ȷ��һ���������Ӧѡ�����������еģ� ��
���ܲ���ܼ��۵����Ƶ���չ����ܵ��ӵ�����״̬
A.�٢ڢۢ�B.�٢ڢ�C.�٢�D.��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��һ��������N2��H2��Ӧ����NH3����ش�
������Ӧ���������ΪE1���������������ΪE2����E1��E2����÷�ӦΪ________(����ȡ����ȡ�)��Ӧ��
����֪��1 mol H��H����1 mol N��H����1 mol N��N���ֱ���Ҫ��������436 kJ��391 kJ��946 kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ________________��
(2) N2H4��H2O2��Ͽ�������ƽ�������֪��16 gҺ̬N2H4������������Ӧ����N2(g)��H2O(l)���ų�310.6 kJ��������2H2O2(l)===O2(g)��2H2O(l)����H����196.4 kJ��mol��1����ӦN2H4(g)��O2(g)===N2(g)��2H2O(l)�Ħ�H��____________kJ��mol��1��N2H4��H2O2��Ӧ����N2(g)��H2O(l)���Ȼ�ѧ����ʽΪ_______________________________________��
(3)ʵ������50 mL 0.50 mol��L��1������50 mLijŨ�ȵ�NaOH��Һ����ͼ��ʾװ���з�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ���װ�����������ԵĴ�������һ����ȱ��һ�ֲ���������������������Ϊ____________��ʵ�����ṩ��0.50 mol��L��1��0.55 mol��L��1����Ũ�ȵ�NaOH��Һ��Ӧѡ��_____________mol��L��1��NaOH��Һ����ʵ�顣

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�ء�
�ش��������⣺
(1) Ԫ��M�ĺ�������Ų�ʽ________ ��Ԫ��L�ĵ����Ų�ͼΪ________ ��
(2)����Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����____________����Ԫ�ط��ű�ʾ����Y��Z��L�ĵ������ɴ�С��˳����______________����Ԫ�ط��ű�ʾ��
(3)Z��X��Ԫ�ذ�ԭ����Ŀ��l��3��2��4���ɷ���A��B��A�ķ��ӿռ乹��Ϊ__________������ԭ�ӵ��ӻ���ʽ��________��A�ĵ���ʽΪ____________��B�ĽṹʽΪ____________��
(4)NO3���Ŀռ乹��Ϊ__________��д��NO3����Ϊ�ȵ������һ�ַ��ӵĻ�ѧʽ_____________��
(5)Ǧ���������γɵ�ij������ľ����ṹ�ǣ�Pb4+���������������㣬Ba2+���ھ������ģ�O2-���ھ���������ģ��û����ﻯѧʽΪ____��ÿ��Ba2+��__��O2-��λ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���������ȷֽ����ɽ���������������������������ɵĶ������������������ʵ���֮��Ϊ8��1�������Ԫ�صĻ��ϼ��ڷ�Ӧ�����еı仯��
A. ���� B. ���� C. ���� D. ��ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ľ̿��һ����������Ũ���ᷴӦ�������֣�4�۵������Ϊ��̽��һ��������NO���ܷ���������Ʒ�Ӧ���Լ���Ӧ��IJ������Ƿ����������ƣ�NaNO2����ijС��ͬѧ�������ͼ��ʾװ�ã��г������������õĽ����Ѿ�ʡ�ԣ���

��1��Ϊ����ɸ�ʵ�飬���߿��ڻ���Ҫ����B��E����װ�ã��������������ӵ�˳��Ϊ��
a��( )( )��( )( )��( )( )�� ( )( )���������ӿڵ���ĸ��ţ������Ӻ���������������ԣ�װ��ҩƷ��Ҫͨ��һ��ʱ��ĵ������ٵμ�Ũ���ᣬ��ȼ�ƾ��ƣ�ͨ�뵪������ҪĿ���� ��
��2��װ��E�й۲쵽����Ҫʵ�������� ��
��3��װ��C�������� ��
��4��ijͬѧ���������Ϻ��������������ᣬ���ʲ��ȶ����������ֽ�����һ��������װ��D�г�ַ�Ӧ�����ʵ��֤��D���Ƿ��������������ɣ� ����д�����鲽�輰����
��5���������������������¿ɽ�I������ΪI2��ͬʱ����NO���÷�Ӧ�������������ƺ����IJⶨ����д���÷�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com