
| Cl2 |
| ����(����) |
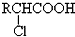 ��
��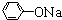 +RCl��NaCl+
+RCl��NaCl+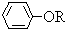
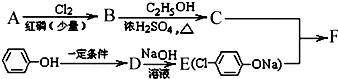
| 8.8 |
| 0.1 |
 ����AΪ
����AΪ ����C�Ľṹ��ʽΪ
����C�Ľṹ��ʽΪ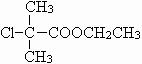 ���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ
���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ ���Լ�aΪ������C��F����ȡ����Ӧ����FΪ
���Լ�aΪ������C��F����ȡ����Ӧ����FΪ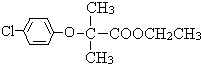 ���ݴ˽��
���ݴ˽��| 8.8 |
| 0.1 |
 ����AΪ
����AΪ ����C�Ľṹ��ʽΪ
����C�Ľṹ��ʽΪ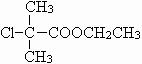 ���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ
���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ ���Լ�aΪ������C��F����ȡ����Ӧ����FΪ
���Լ�aΪ������C��F����ȡ����Ӧ����FΪ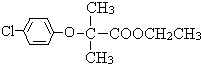 ��
�� ���������ͬ���칹���У�ClCH2CH2CH2COOH��CH3CH��Cl��CH2COOH��CH3CH2CH��Cl��COOH��ClCH2CH��CH3��COOH���ʴ�Ϊ��4��
���������ͬ���칹���У�ClCH2CH2CH2COOH��CH3CH��Cl��CH2COOH��CH3CH2CH��Cl��COOH��ClCH2CH��CH3��COOH���ʴ�Ϊ��4�� ��������ŵ�����Ϊ���ǻ�����ԭ�ӣ��ʴ�Ϊ��
��������ŵ�����Ϊ���ǻ�����ԭ�ӣ��ʴ�Ϊ�� ���ǻ�����ԭ�ӣ�
���ǻ�����ԭ�ӣ� ��
��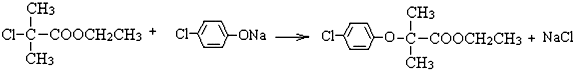 ��
��


 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ڢ� | C���٢� | D���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
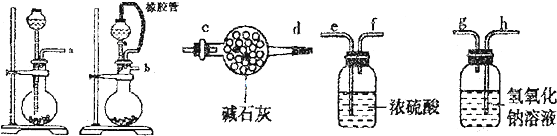
| �ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
| 1 | 1.20 | 16.21 |
| 2 | 3.00 | 18.90 |
| 3 | 4.50 | 19.49 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | ԭ�ӽṹ������ |
| X | Xԭ���ڶ�������ԭ�Ӱ뾶���ϡ��������⣩ |
| Y | ��̬ԭ����3����ͬ���ܼ������ܼ��е�������� |
| Z | ����̬�⻯����������������ˮ���ﻯ���������ӻ����� |
| W | ԭ�Ӻ���s�ܼ�������������p�ܼ��������������γ�W2��W3���ֵ��� |
| T | �ؿǺ�����ߵĽ���Ԫ�� |
| R | R��һ����������������Һ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��Ӧ | A | B | C | D |
| ��H/kJ?mol-1 | 10.5 | 1.80 | -126 | -11.7 |
| ��S/kJ?mol-1?k-1 | 30.0 | -113.0 | 84.0 | -105.0 |
| A����ӦA���κ��¶��¾����Է����� |
| B����ӦB���κ��¶��¾������Է����� |
| C����ӦC�������¶ȸ���170��ʱ�����Է����� |
| D����ӦD���κ��¶��¾������Է����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ca2+��K+��Cl-��CO32- |
| B��Ba2+��Cl-��K+��SO42- |
| C��Fe3+��Cl-��K+��NO3- |
| D��Ag+��NO3-��K+��Cl- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com