
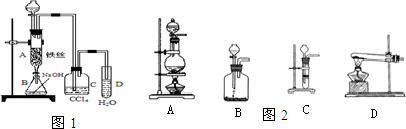
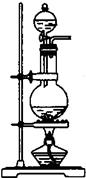
��ͼ��ʵ������ȡ�屽��װ��ͼ��

(1)��װ���Ƿ���ȷ���粻��ȷ����ָ��������________��
(2)����������ȷ��ʵ��װ�ý���ʵ�����ʱ�۲쵽��a��ʢ����������Һ��ʱ��������________����������������ʱ��������________��cƿ��ʢ�ŵ��Լ���________��������________������b��������________��b�ܿڵ�������________��
(3)a�з�Ӧ�Ļ�ѧ����ʽΪ________������Ӧ��ϣ���a�е����ʵ���ʢ����ˮ���ձ��У����ձ��ײ���________ɫ����״Һ�壬����________��________�Ļ������Ƶô������屽��Ӧ��________��Һϴ������������Ӧ�Ļ�ѧ����ʽΪ________�����õ��屽��________ɫҺ�壮

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| Fe |
| Fe |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��������Ϫһ�и߶���ѧ�����п������ƻ�ѧ�Ծ����������� ���ͣ�ʵ����
��15�֣���1��ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
��д��A���л���Ӧ�Ļ�ѧ����ʽ ��
����֪�����л���Ӧ�Ƿ��ȷ�Ӧ���۲쵽A�е������ǣ�
��_____ _________��
�� ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ���� ��д���йصĻ�ѧ����ʽ ��
��C��ʢ��CCl4�������� ��
����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�е���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���______ _____��������______________________��
��2����Ȳ��ʵ�����Ʒ�
�ٷ�Ӧԭ��_____ ____________��
��ѡ����ʵ���ȡʵ��װ��___ ___��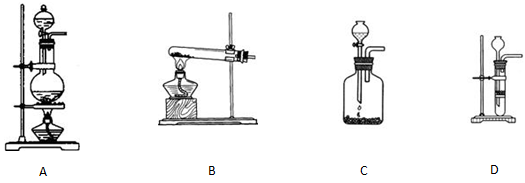
��ʵ���г��ñ���ʳ��ˮ����ˮ��Ŀ����______ __________��
�ܴ�������Ȳ��������ɫ��ζ�����壬�õ�ʯ��ˮ��Ӧ��ȡ����Ȳ��������H2S��PH3���ж����ζ��������____ _______��Һ��ȥ�������塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
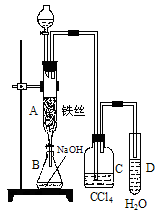
��д��A���л���Ӧ�Ļ�ѧ����ʽ
___________________________________________________
����֪�����л���Ӧ�Ƿ��ȷ�Ӧ���۲쵽A�е�������
_____________________��______________________��
�� ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����_______________________________________________��д���йصĻ�ѧ����ʽ____________________________________________��
��C��ʢ��CCl4��������______________________________________��
����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�е���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���__________________��������___________________________________________��
��2����Ȳ��ʵ�����Ʒ�
�ٷ�Ӧԭ��___________________________________________________��
��ѡ����ʵ���ȡʵ��װ��_______��

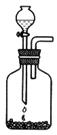 |  | ||
����
������A������������ �������� B������������������������ C�������� ������������ D
��ʵ���г��ñ���ʳ��ˮ����ˮ��Ŀ����___________________________________��
�ܴ�������Ȳ��������ɫ��ζ�����壬�õ�ʯ��ˮ��Ӧ��ȡ����Ȳ��������H2S��PH3���ж����ζ��������________________��Һ��ȥ�������塣
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com