
����Ŀ��ʵ���������������ʵ���Ũ��Ϊ 1 mol/L �� NaOH ��Һ 480 mL��
��1�����Ƹ���Һ��ʵ�鲽�������
a��������Ҫ�������ƹ����������
b�������������ƹ��壻
c�����ձ��е���Һע������ƿ�У�ϴ���ձ��Ͳ���������ϴ��Һȫ��ת��������ƿ�У�
d��������������ˮ�ܽ�����õ��������ƹ��壬��ȴ��
e���Ǻ�ƿ�����������µߵ���ҡ�ȣ�
f������������ƿ�м�����ˮ���̶�����1��2cmʱ������________�μ�����ˮ����Һ����̶������С������������ȷ����˳����________��
��2��ʹ��________mL������ƿ���ƴ���Һ����Ҫ����NaOH���������Ϊ________g��
��3�����в�����ʹ��������ҺŨ��ƫС����________��
A ����ƿ��ԭ����������ˮ
B ת����Һʱ��û��ϴ�Ӳ��������ܽ��õ��ձ�
C ����ҡ�Ⱥ���ʱ����Һ����ڿ̶��ߣ��ּ�ˮ���̶���
D ����ʱ�����ӹ۲�Һ��
���𰸡���ͷ�ι� abdcfe 500 mL 20g BC
��������
(1)��������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ����Ҫ���������ж�ȱ�ٵ�������ע������ƿ���ѡ��Ӧ����������Һ�������������һ�����ʵ���Ũ����Һ��һ�㲽������
(2)����NaOH��Һ480mL��Ӧѡ��500mL����ƿ������m=cVM������Ҫ���ʵ�������
(3)�������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
(1)����һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȣ��õ���������������ƽ��ҩ�ס��ձ���������������ƿ����ͷ�ιܣ�����ʱ����������ƿ�м�����ˮ���̶�����1��2cmʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�����һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȣ�������ȷ�IJ���˳��Ϊ��abdcfe��
(2)����NaOH��Һ480mL��Ӧѡ��500mL����ƿ��ʵ������500mL��Һ����Ҫ������������m=1mol/L��0.5L��40g/mol=20.0g��
(3)A������ƿ��ԭ����������ˮ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��A����
B��ת����Һʱ��û��ϴ�Ӳ��������ܽ��õ��ձ���������ʧ����ҺŨ��ƫ�ͣ���B��ȷ��
C������ҡ�Ⱥ���ʱ����Һ����ڿ̶��ߣ��ּ�ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���C��ȷ��
D������ʱ�۲�Һ�温�ӣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���D����
�ʴ�ΪBC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������23V)���ҹ��ķ��Ԫ�أ��㷺���ڴ���������ҵ���ش��������⣺
��1������Ԫ�����ڱ��е�λ��Ϊ_______����۲�����Ų�ͼΪ____________��
��2������ij��������ľ����ṹ��ͼ1��ʾ��������ʵ��ӵ�е����������Ӹ����ֱ�Ϊ____��_____��
��3��V2O5������SO2ת��ΪSO3�Ĵ�����SO2������Sԭ�Ӽ۲���Ӷ�����__�ԣ����ӵ����幹��Ϊ___��SO3��̬Ϊ�����ӣ��÷�����Sԭ�ӵ��ӻ��������Ϊ____��SO3�������廷״�ṹ��ͼ2��ʾ���ýṹ��Sԭ�ӵ��ӻ��������Ϊ___���ýṹ��S��O���������࣬һ�����Լ140pm����һ�����ԼΪ160pm���϶̵ļ�Ϊ__����ͼ2����ĸ)���÷����к���___��������

��4��V2O5�ܽ���NaOH��Һ�У��ɵõ������ƣ�Na3VO4)�����������ӵ����幹��Ϊ___��Ҳ���Եõ�ƫ�����ƣ��������ӳ���ͼ3��ʾ��������״�ṹ����ƫ�����ƵĻ�ѧʽΪ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(H3PO2)��һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԣ��ش��������⣺
��1��H3PO2��һԪ��ǿ�ᣬд������뷽��ʽ��______________________��
��2��H3PO2��NaH2PO2���ɽ�AgNO3��Һ�е�Ag+ ��ԭΪ�����Ӷ������ڻ�ѧ������
��H3PO2�У���Ԫ�صĻ��ϼ�Ϊ______��
������H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4:1��д���÷�Ӧ�Ļ�ѧ����ʽ�����ڷ���ʽ���õ����ű�ʾ�÷�Ӧ�ĵ���ת��:_______��
��NaH2PO2Ϊ____________ ������Ρ�����ʽ�Ρ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڵ���ʵ�˵����ȷ����
A.���������ˮ��Һ�ܵ���,�ʶ��������ǵ����
B.![]() ��AgCl��ˮ���ܽ�Ⱥ�С,�������������
��AgCl��ˮ���ܽ�Ⱥ�С,�������������
C.�������Һͨ�����ܷ�������
D.��ˮ�еμ�������Ũ�ȵĴ�����Һ��,��Һ��������ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С�����ˮ�ɷֺ����ʽ���̽����ʵ�����£�
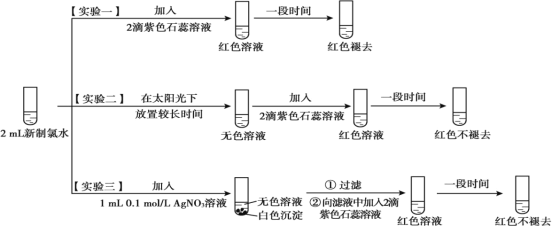
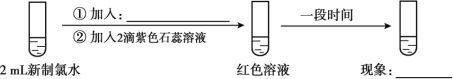
��1����ˮ�ʻ���ɫ��˵�����к���_______________���ѧʽ����
��2��ʵ��һ�������������ˮ�������Ժ�___________ �ԡ�
��3��������ˮ��Ӧ�Ļ�ѧ����ʽΪ_______________ ��
��4���û�ѧ�����ѧ����ʽ��˵��ʵ���������ɫ����ȥ����ԭ�� ______________________��
��5��ʵ����֤����ʵ����������ɫ����ȥ��������Ϊ��ˮ��ϡ�����£����������Լ�������ʵ���ģ�___________��___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������(Fe2O3)���������ᡢ��ī����ҵ����ҵ����һ������������������(��Ҫ�ɷ�ΪFe2O3��Fe3O4������������������)Ϊ��Ҫԭ���Ʊ������һ�ֹ����������£�
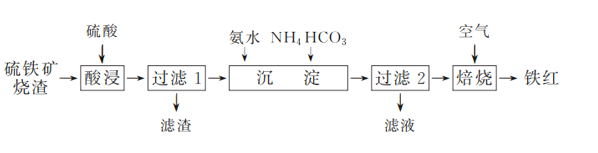
��֪��ijЩ����Ԫ��(��Cu��Fe��Ag��)����������NH3��H2O��OH����SCN�����γɿ���������
(1) ��ҵ������������������������ٽ��������������Ŀ����________��
(2) �������ʱ���������˹���̫���ԭ����________��
(3) ������1��������Һ�к��е���������________��
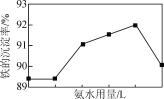
(4) �������������У�����NH4HCO3�������䣬���ij������氱ˮ�����仯����ͼ��ʾ������ˮ��������һ�����ʱ�����ij������½�������ܵ�ԭ����________��
(5) ������2��������������Ҫ�ɷ�ΪFeOOH��FeCO3��������Һ�е���Ҫ������________(�ѧʽ)��
(6) д��FeCO3�ڿ����б�����������Ļ�ѧ����ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һ�ֵ������Һ����ѭ�����������͵�ء�����˵����ȷ����
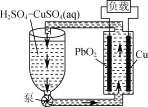
A.Cu�缫Ϊ����
B.PbO2�缫��ӦʽΪPbO2��2e����4H��=Pb2����2H2O
C.�ŵ��ѭ��Һ��H2SO4��CuSO4���ʵ���֮�ȱ�С
D.����Cu����Pb�����·�е���ת�Ʒ���ı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���н�����װ���еĵ��ɼв�������ز��������������У�������Ӧװ��©�������� ��
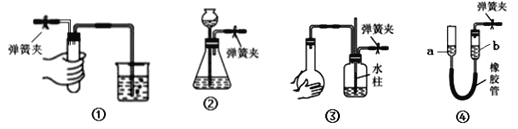
A. װ�â��У�������ס�Թܣ��ձ��г������ݣ��ſ��ֺ������γ�һ���ȶ���ˮ��
B. װ�â��У���©���м���һ����ˮ���γ�ˮ�������ˮ���½�����ƿ��Һ����ƽ��λ��
C. װ�â��У�˫����ס��ƿ���������γ�һ���ȶ���ˮ����˫�ַſ���ˮ����������
D. װ�â��У���a����������һ�θ߶ȣ�a��b����֮��ˮ���γ��ȶ��ĸ߶Ȳ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ����������̼�Ƿ�����ˮ����ʱ��������������Ʒ�Ӧ������������ͨ������ʵ�����֤����

(1)��ͼװ�ã��ڸ�����Թܢ���װ��Na2O2����ͨ��CO2֮ǰ��Ӧ���Ƚ�����(K1��K2)�رպã�Ŀ����____________
(2)�Թܢ��ڵ��Լ�X��________ʱ������K1��K2�������Թܢ�Լ5���Ӻ������ǵ�Сľ�������Թܢ��Һ���ϣ��ɹ۲쵽�����ǵ�Сľ�����ܾ��ҵ�ȼ���������Ң��ڵ���ɫ��ĩδ�����仯�������õĽ�����____________________
(3)�Թܢ����Լ�ΪCO2����ˮ��Һʱ����������ͬ(2)��ͨ��________��������֤��Na2O2�볱ʪ��CO2�ܷ�Ӧ�ҷų�O2��
(4)д�� Na2O2��CO2��Ӧ�Ļ�ѧ����ʽ___________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com