
ЁОЬтФПЁПЂё.ЭМ1ЪЧЪЕбщЪвгУввДМКЭХЈСђЫсжЦввЯЉЕФЗЂЩњзАжУЃЌЭМ2ЪЧввЯЉаджЪЪЕбщзАжУЃЌЧыЛиД№ЃК
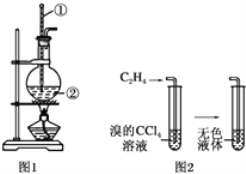
(1)ЭМ1жавЧЦїЂйЁЂЂкЕФУћГЦЗжБ№ЮЊ________ЁЂ________ЁЃ
(2)ЧыаДГіЪЕбщЪвжЦввЯЉЕФЛЏбЇЗНГЬЪНЃК________ЁЃ
(3)ЯђфхЕФЫФТШЛЏЬМШмвКжаЭЈШыввЯЉЃЌШмвКЕФбеЩЋКмПьЭЪШЅЃЌИУЗДгІЪєгк________(ЬюЗДгІРраЭ)ЁЃ
Ђђ.ЪЕбщЪвжЦШЁввЯЉЃЌГЃвђЮТЖШЙ§ИпЖјЪЙввДМКЭХЈСђЫсЗДгІЩњГЩЩйСПЕФЖўбѕЛЏСђЁЃгаШЫЩшМЦЯТСаЪЕбщвдШЗШЯЩЯЪіЛьКЯЦјЬхжагаввЯЉКЭЖўбѕЛЏСђЁЃЧыЛиД№ЃК

(1)ЭМжаЂйЁЂЂкЁЂЂлЁЂЂмзАжУЪЂЗХЕФЪдМСЪЧЯТСажаЕФ(ЧыНЋЯТСагаЙиЪдМСЕФађКХЬюШыЖдгІзАжУФкЃЌПЩжиИДбЁгУ)ЃКЂй_____Ђк_____Ђл______Ђм________
A.ЦЗКьШмвК B.ЧтбѕЛЏФЦШмвК C.ХЈСђЫс D.ЫсадИпУЬЫсМиШмвК
(2)ФмЫЕУїЖўбѕЛЏСђЦјЬхДцдкЕФЯжЯѓЪЧ_______ЁЃ
(3)ЪЙгУзАжУЂкЕФФПЕФЪЧ________ЁЃ
(4)ЪЙгУзАжУЂлЕФФПЕФЪЧ________ЁЃ
(5)бщжЄКЌгаввЯЉЕФЯжЯѓЪЧ_______ЁЃ
ЁОД№АИЁПЮТЖШМЦ дВЕзЩеЦП CH3CH2OH![]() CH2=CH2Ёќ+H2O МгГЩ A B A D ЂйжаЦЗКьШмвКЭЪЩЋ ЮќЪеЖўбѕЛЏСђЦјЬх МьбщЖўбѕЛЏСђЪЧЗёГ§ОЁ ЂлжаЦЗКьВЛЭЪЩЋЃЌЂмжаИпУЬЫсМиЭЪЩЋ
CH2=CH2Ёќ+H2O МгГЩ A B A D ЂйжаЦЗКьШмвКЭЪЩЋ ЮќЪеЖўбѕЛЏСђЦјЬх МьбщЖўбѕЛЏСђЪЧЗёГ§ОЁ ЂлжаЦЗКьВЛЭЪЩЋЃЌЂмжаИпУЬЫсМиЭЪЩЋ
ЁОНтЮіЁП
IIЃЎЮЊСЫжЄУїЛьКЯЦјЬхжагаввЯЉКЭЖўбѕЛЏСђЃЌЯШНЋЛьКЯЦјЬхЭЈЙ§ЦЗКьШмвКЃЌвдДЫМьбщSO2ЃЌдйЭЈЙ§NaOHШмвКГ§ОЁSO2ЃЌХХГ§ЫќЖдМьбщввЯЉЕФИЩШХЃЌМьбщЧАдйДЮгУЦЗКьШмвКМьбщSO2ЪЧЗёГ§ОЁЃЌзюКѓдйЭЈШыЫсадИпУЬЫсМиШмвКжаМьбщввЯЉЁЃ
IЃЎ(1) ЭМ1жавЧЦїЂйЪЧЮТЖШМЦЃЌвЧЦїЂкЪЧдВЕзЩеЦПЃЛ
(2)ЪЕбщЪвжЦБИввЯЉЪЧМгШШввДМКЭХЈСђЫсЕФЛьКЯЮяжС170ЁцжЦЕУЃЌЗДгІЗНГЬЪНЮЊЃКCH3CH2OH![]() CH2=CH2Ёќ+H2OЃЛ
CH2=CH2Ёќ+H2OЃЛ
(3)ЯђфхЕФЫФТШЛЏЬМШмвКжаЭЈШыввЯЉЃЌввЯЉКЭфхЗЂЩњМгГЩЗДгІЩњГЩ1,2-ЖўфхввЭщЃЌЗДгІРраЭЮЊМгГЩЗДгІЃЛ
IIЃЎ(1)гЩгкSO2КЭввЯЉЖМФмЪЙфхЫЎКЭЫсадИпУЬЫсМиШмвКЭЪЩЋЃЌЫљвдЪзЯШвЊМьбщSO2ЃЌРћгУЦЗКьШмвКЃЛЮЊЗРжЙИЩШХввЯЉЕФМьбщЃЌЛЙашвЊГ§ШЅSO2ЃЌРћгУЧтбѕЛЏФЦШмвКЃЌЛЙашвЊдйДЮЭЈЙ§ЦЗКьШмвКРДМьбщSO2ЪЧЗёГ§ОЁЃЌзюКѓНЋЦјЬхЭЈШыЕНЫсадИпУЬЫсМиШмвКРДМьбщввЯЉЁЃЙЪД№АИЮЊЃКAЃЛBЃЛAЃЛDЃЛ
(2)ФмЫЕУїЖўбѕЛЏСђЦјЬхДцдкЕФЯжЯѓЪЧЃКЂйжаЦЗКьШмвКЭЪЩЋЃЛ
(3)зАжУЂкжазАгаNaOHЃЌФПЕФЪЧЃКЮќЪеЖўбѕЛЏСђЦјЬхЃЌвдУтИЩШХввЯЉЕФМьбщЃЛ
(4)зАжУЂлЭЈЙ§ЙлВьЦЗКьШмвКВЛЭЪЩЋРДШЗШЯSO2вбГ§ИЩОЛЃЌД№АИЮЊЃКМьбщЖўбѕЛЏСђЪЧЗёГ§ОЁЃЛ
(5)зАжУЂмЭЈЙ§ИпУЬЫсМиШмвКЭЪЩЋРДМьбщввЯЉЃЌЙЪбщжЄКЌгаввЯЉЕФЯжЯѓЪЧЃКЂлжаЦЗКьВЛЭЪЩЋЃЌЂмжаИпУЬЫсМиЭЪЩЋЁЃ


 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПНЋ 4molA ЦјЬхКЭ 2molB ЦјЬхдк 2L ЕФШнЦїжаЛьКЯЃЌдквЛЖЈЬѕМўЯТЗЂЩњШчЯТЗДгІЃК2A(g) +B(g) 2C(g)ЃЌШєО 2s КѓДяЕНЦНКтЃЌВтЕУ C ЕФХЈЖШЮЊ 0.6molЁЄL-1ЃЌЯжгаЯТСаМИжжЫЕЗЈЃКЂйгУЮяжЪ A ЕФХЈЖШБфЛЏБэЪОЕФЗДгІЫйТЪЮЊ 0.3molЁЄL -1ЁЄs -1ЃЌЂкгУЮяжЪ B ЕФХЈЖШБфЛЏБэЪОЕФЗДгІЫйТЪЮЊ0.6 molЁЄL-1ЁЄs -1ЃЌЂлЦНКтЪБЮяжЪA ЕФзЊЛЏТЪЮЊ70%ЃЌЂмЦНКтЪБЮяжЪB ЕФХЈЖШЮЊ0.7molЁЄL-1ЃЌЦфжае§ШЗЕФЪЧЃЈ ЃЉ
A.ЂйЂлB.ЂйЂмC.ЂкЂлD.ЂлЂм
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШ§ТШЧтЙш(SiHCl3)ЪЧжЦБИЙшЭщЁЂЖрОЇЙшЕФживЊдСЯЁЃЛиД№ЯТСаЮЪЬтЃК
(1)SiHCl3дкГЃЮТГЃбЙЯТЮЊвзЛгЗЂЕФЮоЩЋЭИУївКЬхЃЌгіЫЎЦјЪБЗЂбЬЩњГЩ(HSiO)2OЕШЃЌаДГіИУЗДгІЕФЛЏбЇЗНГЬЪН___ЁЃ
(2)SiHCl3дкДпЛЏМСзїгУЯТЗЂЩњЗДгІЃК
2SiHCl3(g)=SiH2Cl2(g)+SiCl4(g) ІЄH1=48kJЁЄmol-1
3SiH2Cl2(g)=SiH4(g)+2SiHCl3(g) ІЄH2=-30kJЁЄmol-1
дђЗДгІ4SiHCl3(g)=SiH4(g)+3SiCl4(g)ЕФІЄH=___kJЁЄmol-1ЁЃ
(3)ЖдгкЗДгІ2SiHCl3(g)=SiH2Cl2(g)+SiCl4(g)ЃЌВЩгУДѓПзШѕМюадвѕРызгНЛЛЛЪїжЌДпЛЏМСЃЌдк323KКЭ343KЪБSiHCl3ЕФзЊЛЏТЪЫцЪБМфБфЛЏЕФНсЙћШчЭМЫљЪОЁЃ
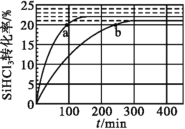
Ђй343KЪБЗДгІЕФЦНКтзЊЛЏТЪІС=___%ЁЃ
ЂкБШНЯaЁЂbДІЗДгІЫйТЪДѓаЁЃКva___vb(ЬюЁАДѓгкЁБЁАаЁгкЁБЛђЁАЕШгкЁБ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП[ЛЏбЇЁЊбЁао3ЃЛЮяжЪНсЙЙгыаджЪ]
УїГЏЁЖЬьЙЄПЊЮяЁЗжагаЪРНчЩЯзюдчЕФЙигкСЖаПММЪѕЕФМЧдиЃЌаПвВЪЧШЫЬхБиашЕФЮЂСПдЊЫиЁЃЛиД№ЯТСаЮЪЬтЃК
(1)ЛљЬЌZnдзгКЫЭтЕФзюИпФмВуЗћКХЪЧ________ЃЌЛљЬЌZn2+зюЭтВуЕчзгХХВМЪНЮЊ________ЁЃ
(2)СђЫсаПШмгкАБЫЎаЮГЩ[Zn(NH3)4]SO4ШмвКЁЃ
ЂйзщГЩ[Zn(NH3)4]SO4ЕФдЊЫижаЃЌГ§ZnЭтЦфгрдЊЫиЕФЕчИКадгЩДѓЕНаЁХХађЮЊ________ЁЃ
ЂкЯђ[Zn(NH3)4]SO4ШмвКжаж№ЕЮЕЮМгNaOHШмвКЃЌЮДГіЯжЛызЧЃЌЦфдвђЪЧ________ЁЃ
ЂлвбжЊ[Zn(NH3)4]2+ЕФПеМфЙЙаЭгы![]() ЯрЭЌЃЌдђдк[Zn(NH3)4]2+жаZn2+ЕФдгЛЏРраЭЮЊ________ЃЌNH3взвКЛЏЕФдвђЪЧ________________________________ЁЃ
ЯрЭЌЃЌдђдк[Zn(NH3)4]2+жаZn2+ЕФдгЛЏРраЭЮЊ________ЃЌNH3взвКЛЏЕФдвђЪЧ________________________________ЁЃ
Ђмдк[Zn(NH3)4]SO4ОЇЬхжаДцдкЕФзїгУСІга________ЁЃ
AЃЎРызгМќ BЃЎМЋадЙВМлМќ CЃЎЧтМќ
DЃЎХфЮЛМќ EЃЎЗЖЕТЛЊСІ FЃЎН№ЪєМќ
(3)ZnOгыZnSНсЙЙЯрЫЦЃЌZnOЕФШлЕуЮЊ1975ЁцЃЌZnSЕФШлЕудМЮЊ1700ЁцЁЃZnOШлЕуБШZnSИпЕФдвђЪЧ________________________________ЁЃ
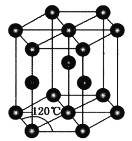
(4)ГЃЮТЯТН№ЪєаПОЇЬхЕФОЇАћЮЊСљЗНзюУмЖбЛ§(ШчЭМЫљЪО)ЃЌШєаПдзгЕФАыОЖЮЊrnmЃЌСљРтжљЕФИпЮЊ![]() ЃЌдђН№ЪєаПОЇЬхЕФПеМфРћгУТЪЪЧ________(гУКЌІаЕФДњЪ§ЪНБэЪО)ЁЃ
ЃЌдђН№ЪєаПОЇЬхЕФПеМфРћгУТЪЪЧ________(гУКЌІаЕФДњЪ§ЪНБэЪО)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЃЈЫЋбЁЃЉЯђФГУмБеШнЦїжаГфШыЕШЮяжЪЕФСПЕФЦјЬхAКЭBЃЌвЛЖЈЮТЖШЯТЗЂЩњЗДгІA(g) + xB(g) ![]() 2C(g)ЃЌДяЕНЦНКтКѓЃЌжЛИФБфЗДгІЕФвЛИіЬѕМўЃЌВтЕУШнЦїжаЮяжЪЕФХЈЖШЁЂЗДгІЫйТЪЫцЪБМфБфЛЏЕФШчЯТЭМЫљЪОЁЃЯТСаЫЕЗЈжае§ШЗЕФЪЧЃЈ ЃЉ
2C(g)ЃЌДяЕНЦНКтКѓЃЌжЛИФБфЗДгІЕФвЛИіЬѕМўЃЌВтЕУШнЦїжаЮяжЪЕФХЈЖШЁЂЗДгІЫйТЪЫцЪБМфБфЛЏЕФШчЯТЭМЫљЪОЁЃЯТСаЫЕЗЈжае§ШЗЕФЪЧЃЈ ЃЉ

AЃЎ30minЪБНЕЕЭЮТЖШЃЌ40minЪБЩ§ИпЮТЖШ
BЃЎ8minЧАAЕФЦНОљЗДгІЫйТЪЮЊ0.08mol/(LЁЄmin)
CЃЎЗДгІЗНГЬЪНжаЕФxЃН1ЃЌе§ЗДгІЮЊЮќШШЗДгІ
DЃЎ20minЁЋ40minМфИУЗДгІЕФЦНКтГЃЪ§ОљЮЊ4
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШ§бѕЛЏЖўюм(Co2O3)ГЃгУгкжЦТЫЙтблОЕЕФЬэМгМСЁЂДпЛЏМСКЭЧПбѕЛЏМСЁЃвдКЌюмЗЯСЯ(жївЊГЩЗжCoOЁЂCo2O3ЃЌКЌгаЩйСПMnO2ЁЂNiOЁЂFe3O4)ЮЊдСЯжЦБИCo2O3ЕФСїГЬШчЯТЃК

(1)баФЅЕФФПЕФЪЧ____________ЁЃТЫдќ1ЕФжївЊГЩЗжЮЊ______________(ЬюЛЏбЇЪН)ЁЃ
(2)ЫсНўЪБЫЋбѕЫЎЕФзїгУга___________ЁЃВЛФмгУбЮЫсДњЬцСђЫсЕФдвђЪЧ_________________ЁЃ
(3)дкЪЕбщЪвРяЃЌнЭШЁВйзївЊгУЕНЕФВЃСЇвЧЦїжївЊга___________ЁЃ
(4)ГСюмЪБЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ_______ЁЃьбЩеЪБЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_______ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаШШЛЏбЇЗНГЬЪНЛђа№Ъіе§ШЗЕФЪЧЃЈ ЃЉ
A.1 molвКЬЌыТдкзуСПбѕЦјжаЭъШЋШМЩеЩњГЩЫЎеєЦјЃЌЗХГі642 kJЕФШШСПЃКN2H4(l)+O2(g)=N2(g)+2H2O(g) ЁїH=+642 kJmol-1
B.C(s)+O2(g)=CO2(g) ІЄH=-393.5 kJ
C.вбжЊЃКH2(g)+![]() O2(g)ЈTH2O(l) ЁїH=-286 kJmol-1ЃЌдђЃК2H2O(l)ЈT2H2(g)+O2(g)ЕФЁїH=+572 kJmol-1
O2(g)ЈTH2O(l) ЁїH=-286 kJmol-1ЃЌдђЃК2H2O(l)ЈT2H2(g)+O2(g)ЕФЁїH=+572 kJmol-1
D.2NaOH(aq)+H2SO4(aq)=Na2SO4(aq)+2H2O(l) ІЄH=-114.6 kJЁЄmol-1(жаКЭШШ)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгЩКЯГЩЦјжЦБИЖўМзУбЃЌЩцМАШчЯТЗДгІЃК
ЃЈiЃЉ2CH3OH(g)![]() C2H4(g)+2H2O(g) ІЄH1
C2H4(g)+2H2O(g) ІЄH1
ЃЈiiЃЉ2CH3OH(g)![]() CH3OCH3(g)+H2O(g) ІЄH2
CH3OCH3(g)+H2O(g) ІЄH2
ФмСПБфЛЏШчЭМЫљЪОЃК
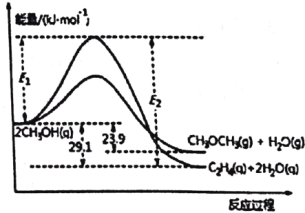
ЯТСаЫЕЗЈе§ШЗЕФЪЧ
A. ІЄH1ЃМІЄH2
B. ЗДгІЃЈiiЃЉЮЊЮќШШЗДгІ
C. C2H4(g)+H2O(g)![]() CH3OCH3(g) ІЄH = -5.2 kJЁЄmolЃ1
CH3OCH3(g) ІЄH = -5.2 kJЁЄmolЃ1
D. ШєдкШнЦїжаМгШыДпЛЏМСЃЌдђE2-E1НЋБфаЁ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙигкЯТСаЫФЗљЭМЯѓгыЖдгІУшЪіЯрЗћКЯЕФЪЧЃЈ ЃЉ
A.![]() ЗДгІжа
ЗДгІжа![]() ЕФЦНКтзЊЛЏТЪЫцЮТЖШКЭбЙЧПЕФБфЛЏ
ЕФЦНКтзЊЛЏТЪЫцЮТЖШКЭбЙЧПЕФБфЛЏ
B. ЫсадИпУЬЫсМиШмвКгыВнЫсЗДгІЪБ
ЫсадИпУЬЫсМиШмвКгыВнЫсЗДгІЪБ![]() ЕФЗДгІЫйТЪЫцЪБМфБфЛЏЕФЭМЯѓ
ЕФЗДгІЫйТЪЫцЪБМфБфЛЏЕФЭМЯѓ
C.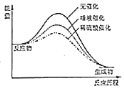 ВЛЭЌДпЛЏМСЖдЕэЗлЫЎНтЗДгІгАЯьЕФЁАФмСП
ВЛЭЌДпЛЏМСЖдЕэЗлЫЎНтЗДгІгАЯьЕФЁАФмСП![]() ЗДгІРњГЬЁБЭМЯѓ
ЗДгІРњГЬЁБЭМЯѓ
D.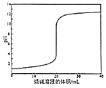 ЩеМюШмвКЕЮЖЈ20mLЕШХЈЖШДзЫсЪБpHЫцМгШыЩеМюШмвКЬхЛ§БфЛЏЕФЭМЯѓ
ЩеМюШмвКЕЮЖЈ20mLЕШХЈЖШДзЫсЪБpHЫцМгШыЩеМюШмвКЬхЛ§БфЛЏЕФЭМЯѓ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com