
��һ����ɫ���壬���ܺ���CaCO3��Na2SO4��KNO3��CuSO4��BaCl2���������е�һ�ֻ��֡��ֽ�������ʵ�飺
��1��ȡ���������ĩ�ӵ�����ˮ�У��õ���ɫ�������ϲ�Ϊ��ɫ��Һ��
��2�����������м�������ϡ���ᣬ��ɫ������ȫ��ʧ���������ݲ�����
��3��ȡ�������е���Һ�μ�Ba(NO3)2��Һ���а�ɫ�������ɣ��ټ���ϡ���ᣬ�������ܡ�
��������ʵ�������жϣ��ð�ɫ������һ������__________________��һ��������________�����ܺ���_____________��
�����֤���ܴ��ڵ�����
һ������CaCO3��Na2SO4����2�֣���һ��������CuSO4��BaCl2����2�֣������ܺ���KNO3����1�֣���
ȡ����������ɫ��Ӧ������ɫ�ܲ������������ɫ��֤����KNO3��2�֣�
����������������ݣ�1�����õ���ɫ�������ϲ�Ϊ��ɫ��Һ����˵����Һ�в�����CuSO4�����г�������ΪBaSO4��CaCO3�����ݣ�2���������ɫ������ʧ��ȷ����������BaSO4ӦΪCaCO3������(3)�ж���Һ�к���SO42-����ԭ�����к���Na2SO4������ȷ������KNO3��������ɫ��Ӧ��������ӣ�ȷ��KNO3�Ƿ���ڡ�
���㣺�������ӹ����й����⡣


 ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ۺ�����ת¯ú��[CO��60��80%����CO2��15��20%������N2��]�����Ṥҵβ���е�SO2�����ܾ���β�������ܻ�ñ��շۣ�Na2S2O4�����䲿�ֹ����������£�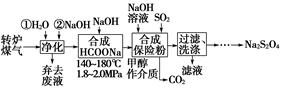
��1��ת¯����ʱ�����ڷ�Ӧ��Fe3C��s����CO2��g��??2CO��g����3Fe��s������ƽ�ⳣ������ʽΪK��________��
��2��ú������ʱ������ˮϴ����NaOH��Һϴ�ӣ���Ŀ����________��
��3������Һ�л��ռ״��IJ���������____________________________��
���ɻ��յ�����������______________________________________��ֻдһ�ֻ�ѧʽ����
��4���ϳɱ��շ۷�Ӧ�Ļ�ѧ����ʽΪ_________________________��
��5�����շۡ�H2O2��������ֽ��Ư�ף�д�����շ��������H2O2����ˮ��Һ�з�Ӧ���������ε����ʵ����ӷ���ʽ��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�弰�仯����㷺Ӧ�����л��ϳɡ���ѧ����������
��1����ˮ�����������Ԫ�صı仯���£�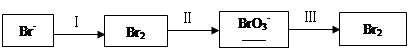
�ٹ��̢�ˮ�Լ��ԣ�����pH��3.5����ͨ��������
��.ͨ��������Ӧ�����ӷ���ʽ��______��
��.����ˮpH�����Cl2�������ʣ���ƽ��ԭ��������ԭ����______��
�ڹ��̢����ȿ�������ϳ�������Ũ̼������Һ���ա���ɲ���ƽ���з���ʽ��
Br2�� Na2CO3��
Na2CO3�� NaBrO3��
NaBrO3�� CO2��
CO2�� ______
______
�۹��̢��������ữ�ɵ�Br2��Na2SO4�Ļ����Һ��
��ͬ�����£����������ữ������������������٣�ԭ����______��
��2��NaBrO3��һ�ַ����Լ����������ữ��NaI��Һ����μ���NaBrO3��Һ��������2.6 mol NaBrO3ʱ����÷�Ӧ����Һ����͵�Ĵ�����ʽ�����ʵ����ֱ�Ϊ��
| ���� | I2 | Br2 | IO3- |
| ���ʵ���/mol | 0.5 | 1.3 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ʣ���NaCl���壻��Һ̬SO2���۴���������ᱵ������ͭ���ƾ���C2H5OH�������ۻ���KCl����NaOH��Һ��
�����������ʻش��������⡣������ţ�
��1��������״̬���ܵ��������������������������
��2������������ʵ�������������������
��3�����ڷǵ���ʣ�������ˮ���ˮ��Һ�ܵ��������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ű�Ҫ��д�����з�Ӧ�����ӷ���ʽ
��������ͭ��������
��̼������Һ�к�������������Һ���
��2�������������ʣ���NaCl���� ��Һ̬HCl ��CaCO3���� ������KCl �����Ǣ�ͭ��CO2��H2SO4��KOH���� ��ˮ�������CH3COOH �Ѿƾ�������ţ�
�������������ܵ������_________________________________��
���������������ڵ���ʵ���_____________________________��
���������������ڷǵ���ʵ���___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͭ��CuFeS2������ͭ���仯�������Ҫԭ��֮һ���������Ʊ������Ļ����
��1��ұ��ͭ�ķ�ӦΪ8CuFeS2��21O2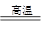 8Cu��4FeO��2Fe2O3��16SO2
8Cu��4FeO��2Fe2O3��16SO2
��CuFeS2��Fe�Ļ��ϼ�Ϊ+2����Ӧ�б���ԭ��Ԫ���� ����Ԫ�ط��ţ���
��2������ұ�������в�������SO2�����д��������������� ������ţ���
a���߿��ŷ� b�������Ʊ�����
c���ô�����Һ������Na2SO3 d����Ũ��������
��3����������أ�K2S2O8������ǿ�����ԣ��ɽ�I������ΪI2��S2O82����2I��=2SO42����I2
ͨ���ı䷴Ӧ;����Fe3���ɴ�������Ӧ���������ӷ���ʽ��ʾFe3����������Ӧ�Ĵ����̡� ��
��
��������ƽ��ÿ��1�֣���
��4�����û�ͭ��ұ��ͭ������¯����Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ
����ϡ�����ȡ¯�������ˡ�
����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յá�
��������Ϣ�ش��������⣺ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ���Լ���ϡ���� ϡ���� KSCN��Һ KMnO4��Һ NaOH��Һ ��ˮ
��ѡ�Լ�Ϊ ��֤��¯���к��е�ʵ������Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������������:
���� ������ ��CO2 ��H2SO4 ��Ba(OH)2 ���ɫ�������������� ��HCl
��1���������������ڵ���ʵ��� ��(�����)
��2�������Һ���μӢߵ���Һ,������������ ��
��3������������������������ˮ��Һ�з�����Ӧ,�����ӷ���ʽΪ:H++OH-=H2O,��÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��װ�����Ӻ������������ձ��м���20mL���ҵ�Ba(OH)2��Һ����ͨ��Դ���μ�ϡ������������
��1�����μ�ϡ����ʱ���ձ��й۲쵽�������ǣ� ��
��2�����ݵ������ǣ� ��ԭ���ǣ� ��
��3��������Ӧ�����ӷ���ʽΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������з�����ij��ѧ��Ӧ����÷�Ӧ�����У������д���Na+��H2O��CN-��ClO-��HCO3-��N2��Cl-�������ӣ�����ClO-��N2�����ʵ�����ʱ��仯��������ͼ��ʾ��������������������: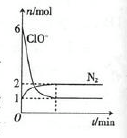
��1��CNһ�е�Ԫ�صĻ��ϼ��� ��
��2���������з�����Ӧ�����ӷ���ʽ�� ���÷�Ӧ������������ (�ѧʽ)��
(3)����������ClO2����ClO-����Һ�ʼ��ԣ���������������N2�����ʵ�����ͬ����ס����������вμӷ�Ӧ�������������ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com