
����Ŀ������������CO�ǻ�����ѧ���о����ȵ���⡣
��1��CO���ڴ���Ȯ����Ⱦ��N2O�������ķ�ӦΪ��N2O(g)+CO(g)![]() CO2(g)+N2(g) ��H���������ʵ�����������£�
CO2(g)+N2(g) ��H���������ʵ�����������£�
���� | N2O(g) | CO(g) | CO2(g) | N2(g) |
�������kJ��mol-1 | 475.5 | 283 | 0 | 393.5 |
�٦�H=______kJ��mol-1���ı����С�������һ��������H�����仯����____����ţ�
A����Ӧ��Ũ�� B������ C����ѧ������
���������������Ӧ������Fe�����������ܷ�Ӧ���������У���һ����Fe+N2O=FeO+N2���ڶ�����______��д��ѧ����ʽ�����ڶ�����Ӧ��Ӱ���ܷ�Ӧ�ﵽƽ������ʱ�䣬�ɴ���֪���ڶ�����Ӧ���____��һ����Ӧ��ܣ���������������С���������������
��2����ʵ���ң����I2O5�ⶨ������CO�ĺ��������ܱ������г���������I2O5��ĩ��һ������CO��������Ӧ��I2O5(g)+5CO(g)![]() 5CO2(g)+I2(s)�����CO��ת������ͼ1��ʾ��
5CO2(g)+I2(s)�����CO��ת������ͼ1��ʾ��

���������a������b���ı�һ���������ı������������______��
���ڴ��¶��£��ÿ��淴Ӧ��ƽ�ⳣ��K=_____���ú�x�Ĵ���ʽ��ʾ����
��3����ҵ�ϣ���CO��H2�ϳ�CH3OH����1L�����ܱ������г���1molCO(g)��nmolH2����250�淢����Ӧ��CO(g)+2H2(g)![]() CH3OH(g)����û��������CH3OH�����������H2�����ʵ����Ĺ�ϵ��ͼ2��ʾ����a��b��c��d���У�CO��ƽ��ת�������ĵ���___��
CH3OH(g)����û��������CH3OH�����������H2�����ʵ����Ĺ�ϵ��ͼ2��ʾ����a��b��c��d���У�CO��ƽ��ת�������ĵ���___��

��4��CO-��������ȼ�ϵ�أ���KOH������ʣ�����ǡ����ȫ����KHCO3ʱֹͣ�ŵ硣д����ʱ�����ĵ缫��Ӧʽ��______��
���𰸡�-365 C FeO++CO=Fe++CO2 С�� ���������������ѹǿ�� 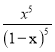 d CO-2e-+3OH-=HCO3-+H2O
d CO-2e-+3OH-=HCO3-+H2O
��������
(1)�١�H=�����������е�������-��Ӧ�������е�����������Ӧ��ֻ����巴Ӧ�Ļ�ѧ�������йأ����¶ȡ�ѹǿ��������ת���ʡ���Ӧ��Ũ�ȵ��أ�
�ڸ��ݴ������壬�ڶ�����Ӧ���ܷ�Ӧ����û��Ӱ�죬˵����һ��������Ӧ�������ܷ�Ӧ���ʣ�
(2)�ٶ��������������ͬ�ķ�Ӧ����ѹ��������Ũ�ȣ������淴Ӧ����ͬ��������������������淴Ӧ����ͬ��������ƽ�ⲻ�ƶ���
�ڸ���ƽ�ⳣ��������㣻
(3)ͼ2�У�b�����ƽ��㣬����H2��CO��Ͷ�ϱȣ�CO��ƽ��ת��������d��CO��ƽ��ת�������
(4)������CO����������Ӧ����KHCO3��
(1)�١�H=�����������е�������-��Ӧ�������е�������=(393.5+0-475.5-283)kJmol-1=-365kJmol-1����Ӧ��ֻ����巴Ӧ�Ļ�ѧ�������йأ����¶ȡ�ѹǿ��������ת���ʡ���Ӧ��Ũ�ȵ��أ��ʴ�ΪC��
�ڸ��ݴ������壬�ڶ�����Ӧ�У��м����(FeO+)����CO����CO2��������ԭ��Fe+��FeO++CO=Fe++CO2���ڶ�����Ӧ���ܷ�Ӧ����û��Ӱ�죬˵����һ��������Ӧ�������ܷ�Ӧ���ʣ��ڶ�����Ӧ���ʴ��ڵ�һ����Ӧ���ʣ���Ӧ����Խ�죬���Խ�ͣ���ڶ�����Ӧ�Ļ��С�ڵ�һ����Ӧ�Ļ�ܣ�
(2)������b������a��ƽ��״̬��ͬ������b��Ӧ���ʽϴ��������������ͬ�ķ�Ӧ����ѹ��������Ũ�ȣ������淴Ӧ����ͬ��������������������淴Ӧ����ͬ��������ƽ�ⲻ�ƶ�������b���ı�һ���������ı�����������Ǽ������(������ѹǿ)��
����CO����ʼŨ��Ϊc(���ڵ������������Ӧ�����ʼ�ղ���)��ƽ��ʱ��c(CO)=(1-x)cmolL-1��c(CO2)=xcmolL-1��K=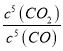 =
=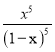 ��
��
(3)ͼ2�У�b�����ƽ��㣬����H2��CO��Ͷ�ϱȣ�CO��ƽ��ת����������a��b��c��d���У�CO��ƽ��ת�������ĵ���d���ʴ�Ϊd��
(4)������CO����������Ӧ����KHCO3�������ĵ缫��Ӧʽ��CO-2e-+3OH-=HCO3-+H2O��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪCaF2��H3BO3(��״�ṹ�����ڵ�H3BO3����ͨ��������)������ͭ���־���Ľṹʾ��ͼ����ش��������⣺


ͼ��ͭ������ͭԭ�Ӷѻ�ģ��
(1)ͼ����ʾ��CaF2��������Ca2������ҵȾ����F����Ϊ________________��ͼ����δ��ŵ�ͭԭ���γɾ������Χ����ڵ�ͭԭ����Ϊ__________________________________��
(2)ͼ����ʾ�����ʽṹ�������ܲ��Ѵ�8���ӽṹ��ԭ����________��H3BO3������Bԭ�Ӹ����뼫�Լ�������Ϊ____________��
(3)����ͭ���кܺõ���չ�ԡ������ԡ������ԣ��Դ�������Ľ�������________���ۡ�
(4)���־������۵���͵���________(�ѧʽ)���侧�������ۻ�ʱ���˷�����֮��������Ϊ____________________________________________________________��
(5)��֪�������������Ca2���˼����Ϊa��10��8cm�����CaF2����ľ���ʾ��ͼ��CaF2������ܶ�Ϊ_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ܱ������н��з�Ӧ��2SO2��O2![]() 2SO3(g)����֪��Ӧ������ijһʱ��SO2��O2��SO3Ũ�ȷֱ�Ϊ0.2 mol��L��1��0.1 mol��L��1��0.2 mol��L��1������Ӧ�ﵽƽ��ʱ�����ܴ��ڵ�������
2SO3(g)����֪��Ӧ������ijһʱ��SO2��O2��SO3Ũ�ȷֱ�Ϊ0.2 mol��L��1��0.1 mol��L��1��0.2 mol��L��1������Ӧ�ﵽƽ��ʱ�����ܴ��ڵ�������
A.SO2Ϊ0.4 mol��L��1��O2Ϊ0.2 mol��L��1B.SO2Ϊ0.3 mol��L��1
C.SO3Ϊ0.4 mol��L��1D.SO2��SO3��Ϊ0.1 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
(1)EԪ�������ڱ��е�λ��Ϊ_________________��д��E������������ˮ������D������������ˮ���ﷴӦ�����ӷ���ʽ________________________________��
(2)��A��B����Ԫ����ɵ�18�������ķ���ʽΪ____________________��
(3)���ⶨA2W2Ϊ��Ԫ���ᣬ�������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ_________________________________________��
(4)Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ�ֻ�����DA���۵�Ϊ800�棬����ˮ��Ӧ������������1molDA��1molE���ʻ�ϼ���������ˮ����ַ�Ӧ����������������_________L(��״����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����н�������A��B��C������ס��ҡ���������D��E��F��G��H������֮���ܷ������·�Ӧ��ת����ϵ����Щ��Ӧ�IJ���ͷ�Ӧ������û��ȫ�������
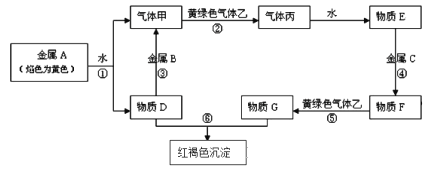
�����������Ϣ�ش��������⣺
(1)д������Ԫ��A�����ڱ��е�λ�ã�____________������D�ĵ���ʽ��____________��
(2)д�����з�Ӧ�����ӷ���ʽ����Ӧ��__________________________________��
��Ӧ��_______________________________________��
(3)��������G�������ӵIJ������� ________________________________________��
(4)ʵ������ȡ����ɫ�����ҵĻ�ѧ����ʽΪ _________________________________���ڸ÷�Ӧ������0.5mol�����ɣ�ת�Ƶ��ӵ����ʵ�����______mol����ȡ��ϣ�������ҽ���D��ˮ��Һ�����գ������ӷ���ʽΪ ______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����״���£���4.48L��NO2��NO��ɵĻ������ͨ��100mL��ˮ�У����������СΪ2.24L��������Һ��������䣬������˵�����������ǣ� ��
A.������Һ�����ʵ����ʵ���Ũ��Ϊ1.0mol��L-1
B.ʣ�������е�Ԫ������Ԫ�ص�����Ϊ7��8
C.ԭ���������NO2��NO�������Ϊ1��1
D.��Ӧ������ת�Ƶĵ�������Ϊ0.1mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ͼ��

���з�Ӧ�з�������ͼ�����(����)
A. N2(g)��3H2(g)![]() 2NH3(g) ��H��0
2NH3(g) ��H��0
B. 2SO3(g)![]() 2SO2(g)��O2(g) ��H��0
2SO2(g)��O2(g) ��H��0
C. 4NH3(g)��5O2(g)![]() 4NO(g)��6H2O(g) ��H��0
4NO(g)��6H2O(g) ��H��0
D. H2(g)��CO(g)![]() C(s)��H2O(g) ��H��0
C(s)��H2O(g) ��H��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������о���Ա������ú��������Ȼ�ѧѭ��ʵ��̫���ܵ�ת����洢��������ͼ��

��1����Ӧ��2H2SO4(l)===2SO2(g)��2H2O(g)��O2(g) ��H1����551 kJ��mol��1
��Ӧ��S(s)��O2(g)===SO2(g) ��H3����297 kJ��mol��1
��Ӧ����Ȼ�ѧ����ʽ��_________��
��2���Է�Ӧ����ijһͶ�ϱ�ʱ������ѹǿ�£�H2SO4��ƽ����ϵ�����ʵ����������¶ȵı仯��ϵ��ͼ��ʾ��p2___p1(����>������<��)���ó��ý��۵�������____��

��3��I��������Ϊˮ��Һ��SO2�绯��Ӧ�Ĵ��������ܵĴ��������¡���������������
��.SO2��4I����4H��===S����2I2��2H2O
��.I2��2H2O��_____===_____��____��2I��
��4��̽����������Ӧ������SO2�绯��Ӧ���ʵĹ�ϵ��ʵ�����£��ֱ�18 mLSO2������Һ����2 mL�����Լ��У��ܱշ��ù۲�����(��֪��I2���ܽ���KI��Һ��)

��B��A�ĶԱ�ʵ�飬��a��_____��
�ڱȽ�A��B��C���ɵó��Ľ�����_______��
��ʵ�������SO2���绯��Ӧ����D>A����Ϣ�������Ӧ���ʽ���ԭ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±��г��ˢ١���ʮ��Ԫ�������ڱ��е�λ�á�
�� ���� | ��A | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | �� | ||
�ش��������⣺
(1)�١��ܰ�ԭ�Ӹ�����1:1 ��ɵķ��ӵĵ���ʽΪ____________________ ���ɢڡ�������Ԫ����ɵ�һ����������ĽṹʽΪ _____________________��
(2)��10��Ԫ���У���ѧ��������õ�Ԫ����_____________(��Ԫ�ط��ţ���ͬ)���õ���������ǿ��ԭ����__________________��ʧ����������ǿ�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ��_________________________��
(3)�û�ѧ����ʽ��ʾ�ں͢�����Ԫ�صķǽ�����ǿ����________________________ ��
(4)Ԫ�آ۵���̬�⻯���Ԫ�آ����̬�⻯���У������Ʊ����� ____________________(�ѧʽ)
(5)Ԫ�آݵ�����������Ӧ��ˮ������Ԫ�آߵ�����������Ӧ��ˮ���ﷴӦ�������ӷ���ʽΪ ______________________________��
(6)Ԫ�� �١��ܡ�������֮������γ��������͵Ļ����д��һ�ֹ��ۻ�����Ļ�ѧʽ��___________________ ��д��һ�����ӻ�����Ļ�ѧʽ��______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com