
����Ŀ����1����֪C(s�����ʯ��+O2(g)==CO2(g)����H= -395.4kJ/mol��C(s��ʯī��+O2(g)==CO2(g)����H= -393.5kJ/mol��
��ʯī�ͽ��ʯ��ȣ�ʯī���ȶ���______���ʯ���ȶ��ԡ�
��ʯī��C-C������______���ʯ��C-C�����ܡ�������������������С������������������
��2����֪H��H���ļ���Ϊ436 kJ��mol��1��Cl��Cl���ļ���Ϊ243 kJ��mol��1��H��Cl���ļ���Ϊ431 kJ��mol��1����H2(g) ��Cl2(g)=2HCl(g)�ķ�Ӧ��Ϊ______��
��3����֪���з�Ӧ�ķ�Ӧ�ȣ�
CH4(g)��H2O(g)��CO(g)��3H2(g) ��H1��+206.2kJ��mol��1
CH4(g)��CO2(g)��2CO(g)��2H2(g) ��H2��-247.4 kJ��mol��1
��CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ______________��
���𰸡����� ���� ��H = -183kJ��mol-1 CH4(g)��2H2O(g)��CO2(g)��4H2(g) ��H��+659.8kJ��mol��1
��������
��1�����ʾ��е�����Խ��Խ�ȶ���
��2�����ݻ�ѧ��Ӧ�оɼ��Ķ������ȣ��¼����γɷ��ȣ�
��3�����ݸ�˹������⣻
��1������֪���ʯ��ʯīȼ��������ͬ�Ķ�����̼ʱ�ͷŵ������࣬����ͬ���Ľ��ʯ���е���������ʯī�����е�����������Խ��Խ�ȶ���ʯī���ȶ���
������Խ�ȶ��������Խ��Խ�ѷ�����Ӧ��ʯī�Ƚ��ʯ�ȶ�������ʯī�е�̼̼���Ƚ��ʯ�еļ��ܴ�
��2����ѧ��Ӧ�оɼ��Ķ������ȣ��¼����γɷ��ȣ���436 kJ/mol+243 kJ/mol-431 kJ/mol![]() 2=-183kJ/mol�����Ȼ�ѧ����ʽΪH2(g) ��Cl2(g)=2HCl(g) ��H = -183kJ/mol��
2=-183kJ/mol�����Ȼ�ѧ����ʽΪH2(g) ��Cl2(g)=2HCl(g) ��H = -183kJ/mol��
��3�����ݸ�˹���ɿ�֪����![]() 2-�ڿɵ�CH4(g)��2H2O(g)=4H2(g)��CO2(g) ��H��+659.8kJ/mol��
2-�ڿɵ�CH4(g)��2H2O(g)=4H2(g)��CO2(g) ��H��+659.8kJ/mol��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.4 g NaOH��1.06 g Na2CO3��ϲ������Һ������Һ�еμ�0.1 mol��L��1ϡ���ᡣ����ͼ������ȷ��ʾ������������������CO2�����ʵ����Ĺ�ϵ����( )
A.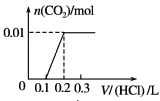 B.
B.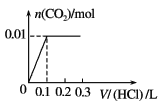
C.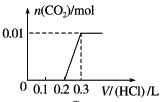 D.
D.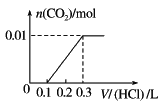
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ԭ���װ�ã�X��YΪ���缫���������ҺΪϡ���ᣬ���·�еĵ���������ͼ��ʾ���Դ�װ�õ�����˵����ȷ���ǣ� ��
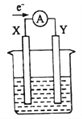
A. ���·�ĵ�������Ϊ��X�����·��Y
B. �����缫�ֱ�ΪZn��ʯī������XΪʯī����YΪZn
C. SO42-����X�缫��Y�缫������������
D. X���Ϸ������ǻ�ԭ��Ӧ��Y���Ϸ�������������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴Ӧ2A(g)+3B(g)![]() 2C(g)+D(g)��ͬ�����µķ�Ӧ���ʣ���Ӧ���������ǣ� ��
2C(g)+D(g)��ͬ�����µķ�Ӧ���ʣ���Ӧ���������ǣ� ��
A. v(A)= 0.5mol/ (L��min)B. v(B)=0.03mol/ (L��s)
C. v(C)=0.35mol/ (L��min)D. v(D)=0.4mol/ (L��min)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܶ��ʻ���ˮ������ζ���������Ļ�����ͼ����ϩ��Ϊԭ����ȡ���������Ĺ���(���ֲ���ͷ�Ӧ��������ȥ)��
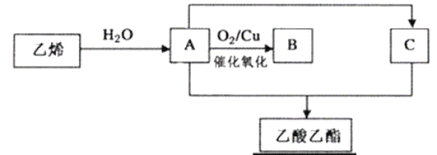
��ش��������⣺
��1��B�Ľṹ��ʽΪ______________��
��2����ϩ��ˮ��Ӧ����A�ķ�Ӧ����Ϊ______________��
��3��A��C��Ӧ�������������Ļ�ѧ����ʽΪ__________���䷴Ӧ����Ϊ__________��
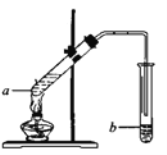
��4��ʵ���ҿ�����ͼװ����ȡ����������
�����Թ�a�мӺ�����Լ�����Ҫ����2��3������Ƭ����������__________��
���Թ�b��ʢ�б���̼������Һ�����ɵ����������ڸ���Һ��_______(��ϡ����¡�)�㣬�÷�Һ�ķ���������ò�Ʒ�������Ҫ������________���Թ�b�еĵ��ܿ�Ӧ��Һ���Ϸ��������뵽Һ�����£���ԭ����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ����ij�л�������A�Ľṹ������������ʵ�飺
��1����NaHCO3��Һ�м���A��������ų���˵��A�к���___________�����ţ�д�ṹ��ʽ����
��2����NaOH��Һ�м�������A������һ��ʱ�����ȴ����HNO3�ữ���ٵμ�AgNO3��Һ����������ɫ������˵����A����___________�����ţ�д���ƣ���
��3������������Mr(A)=153����A��ֻ������Ԫ�أ���A�ķ���ʽΪ___________��
��4���˴Ź���������ʾ��A��������3�֣���ǿ��֮��Ϊ1�U2�U2����A�ṹ��ʽΪ___________��
��5����֪A�ɷ�������ͼ��ʾ��ת����
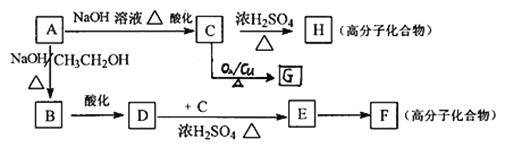
��A��B��D��E�ķ�Ӧ���ͷֱ�Ϊ___________��___________��
��д�����з�Ӧ�Ļ�ѧ����ʽC��H��_________________________��
��C��ͬ���칹���ж��֣�д����C������ͬ�Ĺ����ŵ�C��ͬ���칹��Ľṹ��ʽ��___________________________��д��F�Ľṹ��ʽ��________________________��
��G�������������Һ��Ӧ��ÿ����2.16gAg������G�����ʵ�����__________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.02 mol/L CH3COOH(Ka=1��10��5 mol/L)��Һ��0.01 mol/L NaOH��Һ�������ϣ�������Һ����Ũ�ȹ�ϵ��ȷ����
A. c(CH3COO��)��c(Na��) B. c(CH3COOH)��c(CH3COO��)
C. c(H��)��c(OH��) D. c(CH3COOH)��c(CH3COO��)��0.02 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и����еı�ֵ����2��1����
A. K2S��Һ��c(K+)��c(S2��)֮��
B. pH��Ϊ12���ռ���Һ��Ba(OH)2��Һ�����ʵ���Ũ��֮��
C. ��ͬ�¶���0.2mol/L������Һ��0.1mol/L������Һ�е�c(H+)֮��
D. ����ʱ,��pH��5��H2SO4��Һϡ��1000��,ϡ�ͺ���Һ�е�c(H+)��c (SO42-)֮��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ƽ�������ʾ�����������в��������ķϲ���(��SiO2��Fe2O3��CeO2��FeO������)��ijС���Դ˷ϲ���Ϊԭ�ϣ�������¹������̶���Դ���л��գ��õ�Ce(OH)4��
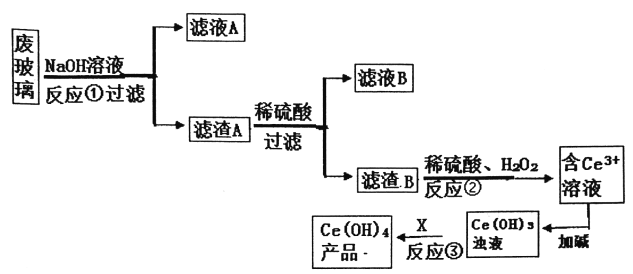
��֪:CeO2������ǿ���ǿ�Ce3+��ˮ�⣬���������£�Ce4+��ǿ�����ԡ�
(1)�ϲ�����NaOH��Һ��ϴǰ����Ҫ���еIJ���________����Ӧ�ٵ����ӷ���ʽ_______��
(2)��Ӧ�ڵ����ӷ�������____________��
(3)Ϊ�˵õ��ϴ���Ce3+��Һ����Ӧ��֮ǰҪ���еIJ�����______��
(4)��Ӧ����Ҫ������Լ�X������_________��
(5)�õζ����ⶨ�Ƶõ�Ce(OH)4��Ʒ���ȡ�
![]()
��FeSO4��Һ�ζ���_____��ָʾ�����ζ��յ������_______������FeSO4��Һ�ڿ�����¶��һ��ʱ����ٽ����еζ������ø�Ce(OH)4��Ʒ����������____(�ƫ����ƫС������Ӱ�족)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com