
ЁОЬтФПЁПФјЪЧживЊЕФКЯН№дЊЫиЃЌПЩжЦзїФјИѕЁЂФјТСЕШКЯН№ЃЌФјвВГЃгУзїгаЛњМгЧтЕФДпЛЏМСМАжЦШЁХфКЯЮяЁЃ
ЃЈ1ЃЉаДГіЛљЬЌCrЕФМђЛЏЕчзгХХВМЪН__________ЃЌCrжаЙВга__________жжВЛЭЌФмМЖЕФЕчзгЁЃ
ЃЈ2ЃЉNi(CO)nгыFe(CO)5ЭЌЪєН№ЪєєЪЛљХфКЯЮяЃЌаЮГЩХфКЯЮяЪБЃЌУПИіCOЬсЙЉвЛЖдЕчзггыН№ЪєдзгаЮГЩХфЮЛМќЃЌбаОПЗЂЯжН№ЪєдзгЕФМлЕчзгКЭCOЬсЙЉЕФЕчзгзмКЭЕШгк18ЁЃ
ЂйNiЁЂCЁЂOЕФЕчИКадгЩДѓЕНаЁЕФЫГађЮЊ____________________ЁЃ
Ђк Ni(CO)nЗжзгжаn=__________ЁЃ
ЂлвбжЊNi2+КЭFe2+ЕФРызгАыОЖЗжБ№ЮЊ69pmКЭ78pmЃЌИљОнбвНЌОЇГіЙцдђШлШкЕФNiOКЭFeOбвНЌдкРфШДЙ§ГЬжаЃЌNiOИќШнвзЯШНсОЇЃЌЪдНтЪЭЦфдвђ____________________ЁЃ
ЃЈ3ЃЉМьЖЈФјЁЂюмЁЂЭюйЕШПЩгУЫЋЧшАЗЃЌЛЏбЇЪНC2H4N4ЃЌЦфНсЙЙМђЪНШчЭМЫљЪОЁЃЫЋЧшАЗЗжзгжаЬМдзгЕФдгЛЏЗНЪНга__________ЃЌЗжзгНсЙЙжаМќФмзюДѓЕФЙВМлМќЪЧ__________ЁЃ
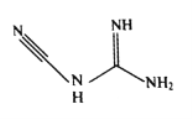
ЃЈ4ЃЉФјЕФОЇЬхНсЙЙЁЂФјТСКЯН№ЕФОЇАћШчЭМЫљЪОЁЃ
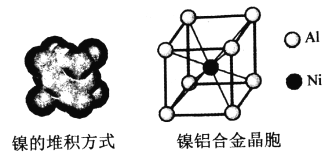
ЂйФјОЇЬхЕФЖбЛ§ЗНЪНЮЊ__________ЁЃ
ЂквбжЊAlЕФЕквЛЁЂЕкЖўЕчРыФмЗжБ№ЮЊЃКl1=578kJ/molЃЌl2=1817kJ/molЃЌМђЪіl2дЖДѓгкl1ЕФдвђ__________________________________________________ЁЃ
ЂлвбжЊЃКФјТСКЯН№УмЖШЮЊdg/cm3ЃЌNAДњБэАЂЗќйЄЕТТоГЃЪ§ЃЌдђТСФјЕФКЫМфОрЮЊ__________ pmЁЃ(гУДњЪ§ЪНБэЪО)
ЁОД№АИЁП [Ar]3d3 6 O>C>Ni 4 Ni2+РызгАыОЖаЁгкFe2+ЃЌNiOжаРызгМќИќЧПЃЌОЇИёФмИќИпЃЌЙЪNiOШлЕуИпгкFeO,бвНЌРфШДЙ§ГЬжаNiOЯШОЇГі spЁЂsp2 C![]() N УцаФСЂЗНзюУмЖбЛ§ЛђccpЛђfcc вђЮЊAlЪЇШЅвЛИіЕчзгКѓзюЭтВуЪЧ3s2,sЪЧШЋГфТњзДЬЌЃЌБШНЯЮШЖЈЃЌЕкЖўИіЕчзгКмФбЪЇШЅЃЌЫљвдI2дЖДѓгкI1
N УцаФСЂЗНзюУмЖбЛ§ЛђccpЛђfcc вђЮЊAlЪЇШЅвЛИіЕчзгКѓзюЭтВуЪЧ3s2,sЪЧШЋГфТњзДЬЌЃЌБШНЯЮШЖЈЃЌЕкЖўИіЕчзгКмФбЪЇШЅЃЌЫљвдI2дЖДѓгкI1 
ЁОНтЮіЁПЃЈ1ЃЉCrЪєгк24КХдЊЫиЃЌИљОнЙЙдьдРэПЩжЊCrЕФКЫЭтЕчзгХХВМЪНЪЧ1s22s22p63s23p63d3ЃЌМђЛЏЮЊ[Ar]3d3ЃЌгЩЛљЬЌКЫЭтЕчзгХХВМЪНПЩжЊЙВга6жжВЛЭЌФмМЖЃЛ
ЃЈ2ЃЉЂйNiЁЂCЁЂOЕФЕчИКадгЩДѓЕНаЁЕФЫГађЮЊO>C>NiЃЛЂк Ni(CO)nЗжзгжаН№ЪєдзгЕФМлЕчзгКЭCOЬсЙЉЕФЕчзгзмКЭЕШгк18ЃЌNiЕФМлЕчзгЪ§ЮЊ10ЃЌвЛИіCOЬсЙЉ2ИіЕчзгЃЌЫљвдnгІИУЪЧ4ЃЛЂлNi2+РызгАыОЖаЁгкFe2+ЃЌNiOжаРызгМќИќЧПЃЌОЇИёФмИќИпЃЌЙЪNiOШлЕуИпгкFeO,бвНЌРфШДЙ§ГЬжаNiOЯШОЇГіЁЃ
ЃЈ3ЃЉИљОнНсЙЙМђЪНЭМЃЌCдзгЕФЛЏбЇМќСЌНгЗНЪНгажБЯпаЭКЭЦНУцШ§НЧаЮСНжжЙЙаЭЃЌгЩДЫЭЦЖЯдгЛЏЗНЪНЮЊspЁЂsp2СНжжЃЌМќФмДѓаЁЫГађЮЊЃКШ§Мќ>ЫЋМќ>ЕЅМќЃЌЙЪЗжзгНсЙЙжаМќФмзюДѓЕФЙВМлМќЪЧC![]() NЁЃ
NЁЃ
ЃЈ4ЃЉЂйИљОнЭМЪОПЩжЊЃЌФјОЇЬхЕФЖбЛ§ЗНЪНЮЊЃКУцаФСЂЗНзюУмЖбЛ§ЛђccpЛђfccЁЃЂкAlЕФЛљЬЌЕчзгХХВМЪНЮЊ1s22s22p63s23p1ЃЌЪЇШЅ3p1ЕФЕчзгБШНЯШнвзЫљвдЕквЛЕчРыФмНЯаЁЃЌЪЇШЅ3p1Кѓ3s2ЮЊШЋГфТњЃЌНЯФбЪЇШЅЕчзгЃЌЫљвдЕкЖўЕчРыФмдЖДѓгкЕквЛЕчРыФмЁЃЂлИљОнОЇАћНсЙЙЃЌвЛИіОЇАћжаКЌга1ИіAlвЛИіNiЃЌЫљвдвЛИіОЇАћЕФжЪСПЮЊЃК ![]() gЃЛОЇАћЕФБпГЄЮЊ
gЃЛОЇАћЕФБпГЄЮЊ![]() cmЃЌТСКЭФјЕФОрРыЮЊОЇАћЖдНЧЯпЕФвЛАыЃЌЫљвдОрРыЮЊ
cmЃЌТСКЭФјЕФОрРыЮЊОЇАћЖдНЧЯпЕФвЛАыЃЌЫљвдОрРыЮЊ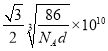 pmЁЃ
pmЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП![]() ЪЧвЛжжгаЛњКЯГЩЕФжаМфЬхЃЌПЩвдЭЈЙ§вдЯТЭООЖРДКЯГЩЃК
ЪЧвЛжжгаЛњКЯГЩЕФжаМфЬхЃЌПЩвдЭЈЙ§вдЯТЭООЖРДКЯГЩЃК

вбжЊЃК(1) 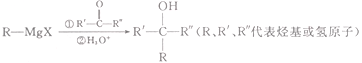
(2) 
(3) ![]()
ЛиД№ЯТСаЮЪЬтЃК
(1)MЕФЗжзгЪНЮЊ__________________ЁЃ
(2)NЕФЯЕЭГУќУћЗЈЕФУћГЦЮЊ_____________________ЁЃ
(3)DжаЙйФмЭХУћГЦЮЊ__________________ЁЃ
(4)гЩAЩњГЩBЕФЛЏбЇЗНГЬЪНЮЊ___________________ЁЃЗДгІРраЭЮЊ________________ЁЃ
(5)HЪЧБШGЩй6ИіЬМдзгЕФGЕФЭЌЯЕЮяЃЌдђHга_________жжЃЌЦфжаКЫДХЙВеёЧтЦзгаШ§зщЮќЪеЗхЕФНсЙЙМђЪНЮЊ___________________ЁЃ
(6)аДГігУТШБНЮЊдСЯжЦБИЛЏКЯЮя ЕФКЯГЩТЗЯпЃК______________________ЁЃ(ЦфЫћЪдМСШЮбЁ)ЁЃ
ЕФКЯГЩТЗЯпЃК______________________ЁЃ(ЦфЫћЪдМСШЮбЁ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдкЭМЃЈЂёЃЉЫљЪОЕФзАжУжаЃЌЩеБжаЪЂЗХЕФЪЧBa(OH)2ШмвКЃЌЕБДгЕЮЖЈЙмжаж№НЅМгШыФГжжШмвКAЪБЃЌШмвКЕФЕМЕчадЕФБфЛЏЧїЪЦШчЭМЃЈЂђЃЉЫљЪОЁЃ


(1)ЕЮМгвКЬхжСЭМЃЈЂђЃЉжаЧњЯпзюЕЭЕуЪБЃЌЕЦХнПЩФмЯЈУ№ЃЌПЩФмЕФдвђЪЧ__________________ЁЃ
(2)ЪдИљОнРызгЗДгІЕФЬиЕуЗжЮіЃЌШмвКAжаКЌгаЕФШмжЪПЩФмЪЧЃЈЬюађКХЃЉ____________ЁЃ
ЂйHCl ЂкH2SO4 ЂлNaHSO4 ЂмNaHCO3
(3)вбжЊ0.1 molЁЄL-1NaHSO4ШмвКжаc(HЃЋ)=0.1 molЁЄL-1ЃЌЧыЛиД№ЯТСаЮЪЬтЃК
ЂйаДГіNaHSO4дкЫЎШмвКжаЕФЕчРыЗНГЬЪН___________________________ЁЃ
ЂкNaHSO4Ъєгк________ЃЈЬюЁАЫсЁБЁЂЁАМюЁБЛђЁАбЮЁБЃЉЁЃ
ЂлЯђNaHSO4ШмвКжаЃЌж№ЕЮМгШыBa(OH)2ШмвКжСжаадЃЌЧыаДГіЗЂЩњЗДгІЕФРызгЗНГЬЪНЃК____________ЃЛдквдЩЯжаадШмвКжаЃЌМЬајЕЮМгBa(OH)2ШмвКЃЌЧыаДГіДЫВНЗДгІЕФРызгЗНГЬЪНЃК_____________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯђ50 mL NaOHШмвКжаж№НЅЭЈШывЛЖЈСПЕФCO2(МйЩшШмвКЬхЛ§ВЛБф)ЃЌЫцКѓШЁДЫШмвК10 mLЃЌНЋЦфЯЁЪЭжС100 mLЃЌВЂЯђДЫЯЁЪЭКѓЕФШмвКжаж№ЕЮМгШы0.1 molЁЄL-1бЮЫсЃЌВњЩњCO2ЦјЬхЕФЬхЛ§(БъзМзДПіЯТ)гыЫљМгШыбЮЫсЕФЬхЛ§ЙиЯЕШчЯТЭМЫљЪОЁЃ

(1) аДГіOAЖЮЫљЗЂЩњЗДгІЕФРызгЗНГЬЪНЃК______________ЁЃ
(2)NaOHдкЮќЪеCO2КѓЃЌЫљЕУШмвКЕФШмжЪЮЊ____ЃЌЦфЮяжЪЕФСПХЈЖШжЎБШЮЊ____ЁЃ
(3)ВњЩњCO2ЕФЬхЛ§(БъзМзДПіЯТ)ЮЊ____ЁЃ
(4)дNaOHШмвКЕФЮяжЪЕФСПХЈЖШЮЊ___ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖЬжмЦкдЊЫиWЁЂXЁЂYЁЂZЁЂQЕФдзгађЪ§вРДЮдіДѓЃЌcЁЂdЁЂeЁЂfЁЂhЪЧгЩетаЉдЊЫизщГЩЕФЖўдЊЛЏКЯЮяЃЌздШЛНчжагВЖШзюДѓЕФЕЅжЪКЭaЖМгЩXзщГЩЃЌbгЩWЁЂYЁЂQШ§жждЊЫизщГЩ0.05mol/LbШмвКЕФpHЮЊ1ЃЌdФмЪЙЦЗКьШмвКЭЪЩЋЃЌeЪЧвКЬхЃЌfЕФбцЩЋЗДгІЮЊЛЦЩЋЃЌЩЯЪіЮяжЪЕФзЊЛЏЙиЯЕШчЭМЫљЪО(ИіБ№ВњЮяТдШЅ)ЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧЃЈ ЃЉ

A. ЖўдЊЛЏКЯЮяЗаЕуЃКe>d>c B. ЧтЛЏЮяЕФЮШЖЈадЃКQ>Y>X
C. дЊЫиЕФЗЧН№ЪєадЃКY>X>W D. дзгАыОЖЕФДѓаЁЃКZ>Q>Y
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвЛЖЈЬѕМўЯТЃЌЯТСаЮяжЪПЩвдЗЂЩњМгОлЗДгІЩњГЩИпЗжзгЛЏКЯЮяЕФЪЧ
A.МзЭщB.ввЭщC.ввДМD.ввЯЉ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаИїзщЮЂСЃФмДѓСПЙВДцЃЌЕБМгШыЯргІЪдМСКѓЛсЗЂЩњЛЏбЇБфЛЏЃЌЧвЗЂЩњЗДгІЕФРызгЗНГЬЪНЪщаДе§ШЗЕФЪЧ( )
бЁЯю | ЮЂСЃзщЃЈЫЎШмвКЃЉ | ЫљМгЪдМС | РызгЗНГЬЪН |
A | H+ЁЂ NaЃЋЁЂ | FeЗл | Fe+H+=Fe3++H2Ёќ |
B | NaЃЋЁЂClЃЁЂ | Н№ЪєФЦ | 2Na+2H2O=2NaЃЋ+2OH-+H2Ёќ |
C | NH4+ЁЂH+ЁЂCH3COO- | бѕЛЏЬњ | 6H++Fe2O3=2Fe3++3H2O |
D | Ca2ЃЋЁЂOH-ЁЂClЃ | ЭЈШыЙ§СПCO2 | OH-+CO2= |
A. A B. B C. C D. D
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПКЯГЩЦј(COКЭH2)ЪЧФПЧАЛЏЙЄГЃгУЕФдСЯЃЌЯТУцЪЧгУМзЭщжЦБИКЯГЩЦјЕФСНжжЗНЗЈЃК
Ђй CH4(g)+H2O(g)![]() CO(g)+3H2(g) ІЄH1=+216 kJЁЄmol-1ЃЛ
CO(g)+3H2(g) ІЄH1=+216 kJЁЄmol-1ЃЛ
Ђк 2CH4(g)+O2(g) ![]() 2CO(g)+4H2(g) ІЄH2=-72 kJЁЄmol-1ЁЃ
2CO(g)+4H2(g) ІЄH2=-72 kJЁЄmol-1ЁЃ
ЦфжавЛИіЗДгІЕФЗДгІЙ§ГЬгыФмСПБфЛЏЙиЯЕШчЭМЫљЪОЁЃдђЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ

A. E1БэЪО2CH4(g)+O2(g) ![]() 2CO(g)+4H2(g)ЕФЛюЛЏФм
2CO(g)+4H2(g)ЕФЛюЛЏФм
B. E2БэЪОCH4(g)+H2O(g)![]() CO(g)+3H2(g)ЕФЛюЛЏФм
CO(g)+3H2(g)ЕФЛюЛЏФм
C. ИУЭМЪОЮЊЗДгІЂкЕФЗДгІЙ§ГЬгыФмСПБфЛЏЪОвтЭМ
D. вЛАуЧщПіЯТЃЌМгШыДпЛЏМСЃЌМШФмНЕЕЭE1ЃЌвВФмНЕЕЭE2ЃЌЕЋВЛФмИФБфE1гыE2ЕФВюжЕ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЁЊЖЈЬѕМўЯТЃЌCO2(g)+3H2(g)![]() CH3OH (g)+H2O(g) ЁїH=Ѓ57.3 kJ/molЃЌЭљ 2L КуШнУмБеШнЦїжаГфШы 1 mol CO2КЭ3 mol H2ЃЌдкВЛЭЌДпЛЏМСзїгУЯТЗЂЩњЗДгІЂйЁЂЗДгІЂкгыЗДгІЂлЃЌЯрЭЌЪБМфФкCO2ЕФзЊЛЏТЪЫцЮТЖШБфЛЏШчЯТЭМЫљЪОЃЌbЕуЗДгІДяЕНЦНКтзДЬЌЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ
CH3OH (g)+H2O(g) ЁїH=Ѓ57.3 kJ/molЃЌЭљ 2L КуШнУмБеШнЦїжаГфШы 1 mol CO2КЭ3 mol H2ЃЌдкВЛЭЌДпЛЏМСзїгУЯТЗЂЩњЗДгІЂйЁЂЗДгІЂкгыЗДгІЂлЃЌЯрЭЌЪБМфФкCO2ЕФзЊЛЏТЪЫцЮТЖШБфЛЏШчЯТЭМЫљЪОЃЌbЕуЗДгІДяЕНЦНКтзДЬЌЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ
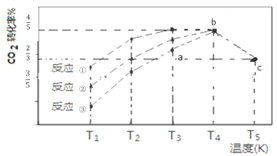
A. a Еу v(е§)>v(ФцЃЉ
B. bЕуЗДгІЗХШШ53.7 kJ
C. ДпЛЏМСаЇЙћзюМбЕФЗДгІЪЧЂл
D. cЕуЪБИУЗДгІЕФЦНКтГЃЪ§K=4/3(mol-2![]() L-2)
L-2)
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com