
��10�֣�����һ���Σ���A��B��C��D��E���ֶ�����Ԫ��Ԫ����ɡ�������ˮ��ɵ�����������ӣ����к�����A��B�γɵ�10���������ӡ�AԪ��ԭ�Ӻ�����������E����1��D��E����ͬ���塣�ü�������ʵ�飺
��ȡ�����ľ�����������ˮ�����Һ��
��ȡ��������Һ���Թ��У������м���ϡ���ᣬ�ټ���BaCl2��Һ�����ְ�ɫ������
��ȡ��������Һ���Թ�����ε���NaOH��Һ�����ɳ��������������NaOH��Һ�������ϵ��ͼ��ʾ��
��ȡ��������Һ���Թ��У��������NaOH��Һ�����ȣ�
�ش��������⣺
��1�� D�����ڱ��е�λ�� д��������AB3�ĵ���ʽ
��2�����ⶨ�����Ħ������Ϊ453 g/mol�����������Ӻ����������ʵ���֮��1:1����1 mol �����к���12 mol�ᾧˮ�������Ļ�ѧʽΪ ��
��3��ʵ����и���ͼ���V(Oa):V(ab):V(bc)= ��
��4��ʵ��������ӷ���ʽ�� ��
(l) ��3���ڢ�A�� ��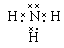 ����2��NH4Al(SO4)2��12H2O[��AlNH4(SO4)2��12H2O] ����3��3:1:1��
����2��NH4Al(SO4)2��12H2O[��AlNH4(SO4)2��12H2O] ����3��3:1:1��
��4�� NH4++Al3++5OH�� NH3��+Al(OH)4��+H2O ��NH4++Al3++5OH��
NH3��+Al(OH)4��+H2O ��NH4++Al3++5OH�� NH3��+AlO2��+3H2O��
NH3��+AlO2��+3H2O��
������������������������֪��A��NԪ�أ�B��HԪ�أ�C��AlԪ�أ�D��SԪ�أ�E��OԪ�ء���1�� D�����ڱ��е�λ����λ�ڵ������ڵڢ�A�壻������AB3�ĵ���ʽ��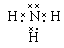 ����2������Ԫ���γɵĻ�������ص㼰���ε���Է���������֪�û�������NH4Al(SO4)2��12H2O[��AlNH4(SO4)2��12H2O] ����3�������ڸ����У�n(Al3+):n(NH4+):n(SO42-)=1:1:2.��û��������Һ�м���NaOH��Һ�����ȷ�����Ӧ��Al3����3OH-��Al(OH)3����Ȼ������Ӧ��NH
����2������Ԫ���γɵĻ�������ص㼰���ε���Է���������֪�û�������NH4Al(SO4)2��12H2O[��AlNH4(SO4)2��12H2O] ����3�������ڸ����У�n(Al3+):n(NH4+):n(SO42-)=1:1:2.��û��������Һ�м���NaOH��Һ�����ȷ�����Ӧ��Al3����3OH-��Al(OH)3����Ȼ������Ӧ��NH + OH-��NH3��H2O���������Ӧ��Al(OH)3+ OH-��AlO2-+ 2H2O�������ʵ����и���ͼ���V(Oa):V(ab):V(bc)=3:1:1����4����������������������������ܹ��������ǿ�����Ӧ������ʵ��������ӷ���ʽ��NH4++Al3++5OH��
+ OH-��NH3��H2O���������Ӧ��Al(OH)3+ OH-��AlO2-+ 2H2O�������ʵ����и���ͼ���V(Oa):V(ab):V(bc)=3:1:1����4����������������������������ܹ��������ǿ�����Ӧ������ʵ��������ӷ���ʽ��NH4++Al3++5OH�� NH3��+Al(OH)4��+H2O ��NH4++Al3++5OH��
NH3��+Al(OH)4��+H2O ��NH4++Al3++5OH�� NH3��+AlO2��+3H2O��
NH3��+AlO2��+3H2O��
���㣺����Ԫ�ؼ���������ƶϡ�ʵ������ķ�������ѧ����ʽ�����ӷ���ʽ��������ĵ���ʽ����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������������ˮ��Һ�д��������һ����
| A��Fe3+��HCO3����Cl����SCN�� | B��Ba2+��NO3����SO32����H+ |
| C��Mg2+��NH4+��Br����OH�� | D��Na+��Cu2+��SO42����Cl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ӷ���ʽ������ȷ����
| A������������ˮ�Ʊ������Cl2��H2O��2H����Cl����ClO�� |
| B����Fe(OH)2�м���������ϡHNO3��Fe(OH)2��2H����Fe2����2H2O |
| C����̼�������Һ�м�������������������Һ��NH4+��OH����NH3��H2O |
| D����������Һ�м������������������Һ��Al3����2SO42����2Ba2����4OH����2BaSO4����AlO2-��2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ����ҵ�ϳ�����������Ҫ�ɷ�ΪAl2O3�������� SiO2��Fe2O3���ʣ��������������
NH4Al(SO4)2?12H2O���乤������ͼ���£�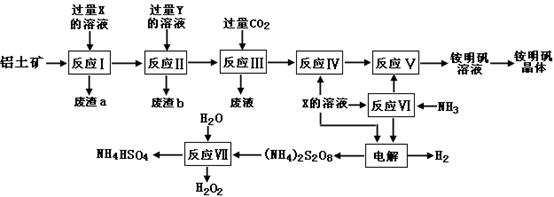
��1������a��b �ijɷֱַ��ǣ�_________��_____________����д���ƣ�
��2������ͼ��X�Ļ�ѧʽΪ��_______________��
��3����Ӧ������ӷ���ʽΪ��_________________________________________�����������Һ�л�����������IJ�������Ϊ����������ƣ�_________����ȴ�ᾧ������ϴ�ӡ�
��4���������[(NH4)2S2O8]�ڹ�ҵ�������й㷺����;��������Ϊ��������Ư�����㷺���������ع�ҵ���������ۺϵ�����������ά��ҵ���ѽ������������Ĺ����������ö��Ե缫���X�뷴Ӧ���������ʵĻ����Һ���Եõ�������李�
д��������Ӧʽ��________________________ ____��
��5����Ӧ���Ļ�ѧ����ʽΪ��_________________________ _____________��
NH4HSO4��Һ������Ũ���ɴ�С˳��Ϊ��__________________________ _��
��6�������������Һ����μ�������������Һ�������ܷ����ķ�Ӧ�� ����ѡ����ĸ��
| A��4NH4Al(SO4)2+3Ba(OH)2��2(NH4)2SO4+3BaSO4��+ Al2 (SO4)3+2Al(OH)3�� |
| B��2NH4Al(SO4)2+4Ba(OH)2��(NH4)2SO4+3BaSO4��+Ba(AlO2)2 |
| C��2NH4Al(SO4)2+3Ba(OH)2��(NH4)2SO4+3BaSO4��+2Al(OH)3�� |
| D��NH4Al(SO4)2+2Ba(OH)2��NH3��H2O+2BaSO4��+ Al(OH)3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣����������ҹ���������̼���о����ش��չ���绡���ϳɵ�̼���ܣ������д������ʡ���̼������������̼���������������������ᴿ���䷴Ӧ�Ļ�ѧ����ʽΪ:
C+ K2Cr2O7 + H2SO4(ϡ) CO2��+ Cr2 (SO4) 3+ + .
CO2��+ Cr2 (SO4) 3+ + .
��1���˷�Ӧ���������� ����������Ԫ���� ��
��2����ɲ���ƽ������Ӧ�Ļ�ѧ����ʽ��
��3��H2SO4��������Ӧ�б��ֳ����������� ����ѡ���ţ�
| A������ | B�������� | C����ˮ�� | D����ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������I��II����ȷ���������ϵ����
| ѡ�� | ����I | ����II |
| A | KNO3���ܽ�ȴ� | ���ؽᾧ����ȥKNO3�л��е�NaCl |
| B | BaSO4�������� | �������BaCl2��Һ����SO42- |
| C | NH3��ʹ��̪��Һ��� | NH3�����������Ȫʵ�� |
| D | Ca(OH)2���Ƴɳ���ʯ��ˮ | ������2.0 mol?L-1��Ca(OH)2��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�����ʯ����Ҫ�ɷ���K2SO4��Al2(SO4)3��2Al2O3��6H2O������������Fe2O3����������ʯ����ȡ������Al2O3��K2FeO4��H2SO4�Ĺ���������ͼ��ʾ��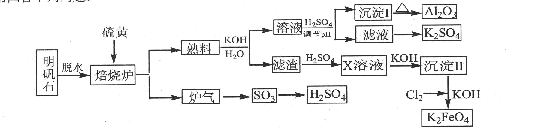
��ش��������⣺
��1������¯�з�����Ӧ�Ļ�ѧ����ʽΪ2Al2(SO4) 2Al2O3+6SO2��+3O2����������102gAl2O3��ת�Ƶĵ�����ĿΪ .
2Al2O3+6SO2��+3O2����������102gAl2O3��ת�Ƶĵ�����ĿΪ .
��2�����ɳ���I�����ӷ���ʽΪ ������II�Ļ�ѧʽΪ ��
��3����Һ�����������pH���ˣ�ϴ�ӣ��ɵó���I��֤������I��ϴ�Ӹɾ���ʵ������������� ��
��4��д��Cl2������������ΪK2FeO4�Ļ�ѧ����ʽ�� ��
��5������Һ�еõ�K2SO4����ķ����� ��
��6�� K2FeO4Ҳ������Ϊ�缫��ͨ�����Ũ��KOH��Һ����ȡ��д����ⷨ��ȡK2FeO4��
������Ӧʽ�� .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ����ѡ��2����ѧ�뼼������15�֣�
��ˮAlCl3���������������л��ϳɵĴ����ȡ���ҵ����������A12O3��Fe2O3��Ϊԭ���Ʊ���ˮAlCl3�Ĺ����������¡�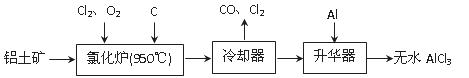
��1���Ȼ�¯��Al2O3��C12��C��Ӧ�Ļ�ѧ����ʽΪ ��
��2����Na2SO3��Һ�ɳ�ȥ��ȴ���ų�β���е�Cl2���˷�Ӧ�����ӷ���ʽΪ ��
��3������������Ҫ����AlCl3��FeCl3�����������Al���������� ��
��4��Ϊ�ⶨ�Ƶõ���ˮAlCl3��Ʒ��������FeCl3���Ĵ��ȣ���ȡ16.25 g��ˮAlCl3��Ʒ�����ڹ�����NaOH��Һ�����˳�����������ᆳϴ�ӡ����ա���ȴ�����أ�������������Ϊ0.32 g��
��д�����ӹ������漰�����ӷ���ʽ �� ��
��AlCl3��Ʒ�Ĵ���Ϊ ��
��5����ҵ����һ��������Ϊԭ���Ʊ���ˮAlCl3�����У����һ������AlCl3��6H2O��ˮ�Ʊ���ˮAlCl3��ʵ����һ���ķ����� ��
��6�������к���һ����̼�����Fe3C������ҵ��Ҫ�ⶨ̼Ԫ�ص�������������һ�����������Ŀ��������գ������д��ԵĹ��壬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ɫ��Һ����Na����Ag����Ba2����Al3����AlO2����MnO4����CO32����SO42����SiO32���е���
�������(������ˮ�ĵ���)��ȡ����Һ��������ʵ�飺
��.ȡ������Һ��������������ᣬ���������ɣ����õ���ɫ��Һ��
��.�ڢ�������Һ�м��������NH4HCO3��Һ�����������ɣ�ͬʱ������ɫ�����ף�
��.�ڢ�������Һ�м��������Ba(OH)2��Һ�����ȣ�Ҳ���������ɣ�ͬʱ������ɫ�����ҡ�
��ش��������⣺
(1)��ʵ����֪ԭ��Һ��һ�������е�������________��һ�����е�������________________��
(2)��ʵ����֪ԭ��Һ�л�һ�����е�������________�����ɼ����ӷ���ʽΪ________________��
(3)ʵ��������ɰ�ɫ�����ҵ����ӷ���ʽΪ________________��
(4)ԭ��Һ�л����ܴ��ڵ�������________����������ӵķ�����_________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com