
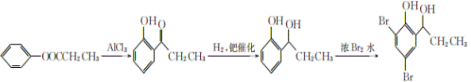
����Ŀ���α������һ������Ѫ�����ŵĽ�Ѫѹҩ�һ�ֺϳ��α�����м���G�IJ����������£�

��֪���������Ľṹ��ʽΪ![]() ��
��
��ش��������⣺
��1��A��������______��B�����������ŵ�������______��
��2����Ӧ�ݵĻ�ѧ����ʽΪ______���÷�Ӧ�ķ�Ӧ������______��
��3��G�ķ���ʽΪ______��
��4��д����������������E��ͬ���칹��Ľṹ��ʽ��______��______��
������ֻ������ȡ����
��˴Ź�������ͼ��ֻ��4�����շ�
��.1mol������������NaHCO3��Һ��Ӧ����2molCO2
��5����������֪ʶ����������Ϣ��д����![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�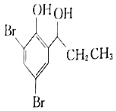 �ĺϳ�·������ͼ�����Լ���ѡ��______���ϳ�·������ͼʾ�����£�CH3CH2Br
�ĺϳ�·������ͼ�����Լ���ѡ��______���ϳ�·������ͼʾ�����£�CH3CH2Br![]() CH3CH2OH
CH3CH2OH![]() CH3COOCH2CH3
CH3COOCH2CH3
���𰸡��Է����ӣ�4-�����ӣ� ��ԭ�ӡ����� 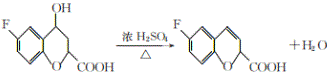 ��ȥ��Ӧ C10H9O3F
��ȥ��Ӧ C10H9O3F 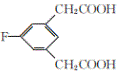
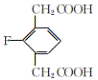
��������
(1)��ԭ�����ǻ��ڱ����Ķ�λ�ϣ�����Ϊ���Է����ӣ�4-�����ӣ���B�к��еĹ�����Ϊ��������ԭ�ӣ�
��2��ͨ���Ա�E��G��E�еĴ��ǻ���Ϊ��ԭ�ӣ����Ϊ���ǻ�����ȥ��Ӧ��
��3������G�к��б������Ȼ���֧������3��̼ԭ�ӣ������ʽΪ��C10H9O3F��
��4��E�ķ���ʽΪ��C10H9O4F�� 1mol������������ NaHCO3��Һ��Ӧ����2 mol CO2����ͬ���칹���к���2���Ȼ����˴Ź�������ͼ��ֻ��4�����շ壬������ֻ������ȡ��������ȡ����Ϊ�Գƽṹ����Ϊ�� ��
�� ��
��
��5����֪��������ˮ��Ӧ����2��4��6-���屽�ӣ���![]() ������ԭ��ǰӦ�Ƚ�������Тڵķ�Ӧ��
������ԭ��ǰӦ�Ƚ�������Тڵķ�Ӧ��
(1)��ԭ�����ǻ��ڱ����Ķ�λ�ϣ�����Ϊ���Է����ӣ�4-�����ӣ���B�к��еĹ�����Ϊ��������ԭ�ӣ�
��2��ͨ���Ա�E��G��E�еĴ��ǻ���Ϊ��ԭ�ӣ����Ϊ���ǻ�����ȥ��Ӧ����ѧ����ʽΪ�� ����Ӧ����Ϊ����ȥ��Ӧ��
����Ӧ����Ϊ����ȥ��Ӧ��
��3������G�к��б������Ȼ���֧������3��̼ԭ�ӣ������ʽΪ��C10H9O3F��
��4��E�ķ���ʽΪ��C10H9O4F�� 1mol������������ NaHCO3��Һ��Ӧ����2 mol CO2����ͬ���칹���к���2���Ȼ����˴Ź�������ͼ��ֻ��4�����շ壬������ֻ������ȡ��������ȡ����Ϊ�Գƽṹ����Ϊ�� ��
�� ��
��
��5����֪��������ˮ��Ӧ����2��4��6-���屽�ӣ���![]() ������ԭ��ǰӦ�Ƚ�������Тڵķ�Ӧ���ʷ�Ӧ����Ϊ��
������ԭ��ǰӦ�Ƚ�������Тڵķ�Ӧ���ʷ�Ӧ����Ϊ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������( NaBiO3 )��һ��������ˮ��ǿ���������ڸ�����ҵ�г�������Ԫ�صķ����ⶨ��ij�о�С���ø�ѡ���Ļ����(��Ҫ�ɷ���Bi2S3����������Bi2O3��SiO2������)�Ʊ������ƣ����������£�

��ش��������⣺
(1)Ϊ���������ȡ����ԭ�ϵĽ����ʣ����Բ�ȡ�Ĵ�ʩ��_________________(дһ�ּ���) ��
(2)����ȡ��ʱͨ������FeCl3��Һ��Ũ���ᣬ�����м������Ũ�����Ŀ����_____�����������ijɷ���____________(�ѧʽ)��
(3)����������Ӧ�����ӷ���ʽΪ__________________________________________________��
(4)������������ʱ���ð�ˮ����pH��6��ͨ������˵������ʱ��Һ�е�Bi3+�Ƿ���ȫ������____________________(��֪��Bi(OH)3���ܶȻ�Ksp=3��10-32) ��
(5)�����������˲�����ѵ������ȡ����ʵ��¶��⣬����Ϊ����Ҫ���Ƶ�������________��
(6)��֪����������Һ��NaBiO3��Mn2+����ΪMnO4-������÷�Ӧ�����ӷ���ʽ��__________��
(7)ij���������Ԫ�ص���������Ϊ20.90%����100�ָû������ȫ�������������õ�25.00��NaBiO3���������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȥ�����л��е���ϩ��������ͨ��ʢ��������һ���Լ���ϴ��ƿ( ��)
A. ����ʯ��ˮ��Ũ����B. ����KMnO4��Һ��Ũ����
C. ��ˮ��Ũ����D. Ũ���ᡢNaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D�������ʾ�������ˮ���ֱ��������������Һ���������ʵ���Ũ��������
A. 0.1 moL D��������100 mL 2.5 mol��L-1��D��Һ��
B. 9.2 g B���ʣ�ʽ��Ϊ46������ˮ���100 mL��Һ
C. 1.806��1023��C�ķ�������ˮ���100 mL��Һ
D. 10 mL 5mol��L-1��A��Һ��ˮ���Ƴ�100 mL��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�ء�
�ش��������⣺
(1)L������Ϊ__________________________������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳��Ϊ______________________________(��Ԫ�ط��ű�ʾ)��
(2)Z��X��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���A��B����ҵ�ϳ�A�Ļ�ѧ����ʽΪ______________________________��B�ĽṹʽΪ_____________________���ڱ�״���£���A����һ��������ƿ������ƿ������ˮ�У�ƿ��Һ��������(�������ʲ���ɢ)�����ƿ����Һ�����ʵ����ʵ���Ũ��Ϊ________(��ȷ��0.001)��
(3)��(Se)������������Ԫ�أ���Lͬһ���壬Seԭ�ӱ�Lԭ�Ӷ��������Ӳ㣬��������������Ӧˮ����Ļ�ѧʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��TMB��̼�����Ԫ����ɣ�����Է�������Ϊ240����һ������ָ�Ƽ���ɫԭ�Լ���������ȡ��ǿ�°����������������°��Ե�������������Ӧ�����ٴ����鷨ҽ�����������Ƽ�������������ij�о�С��������ȼ�շ��ⶨTMB�ķ���ʽ(��Ԫ��ת��ΪN2)ʵ��װ����ͼ��ʾ���ش��������⣺
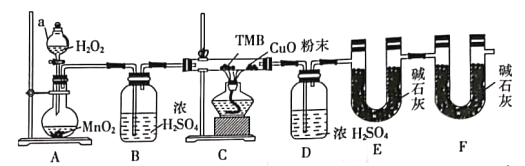
(1)ʵ��װ�������õ�װ�м�ʯ�ҵĸ���ܣ�����F����Ŀ����______________________������a��������___________��
(2)������˫��ˮ����a�У�Բ����ƿ��װ��MnO2���壬��ͼ���Ӻ�װ�á�
��A�з�����Ӧ�Ļ�ѧ����ʽΪ______________________��
�ڴ�B��D�����ܿ��о�������ʱ���ٵ�ȼC���ƾ��ƣ�ԭ����______________________��
(3)װ��C��CuO��ĩ������Ϊ_________________________________��
(4)��ʵ��___________(������Ҫ����������Ҫ��)β����������ԭ����____________________��
(5)����״̬�£���4.80gTMB��Ʒ��ȫ��������ȼC���ƾ��ƣ�ʵ�����ʱ���D����3.60g��E����14.08g����TMB�ķ���ʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1���������˻ᡰ��������������ܴ����Դ�ڱ����ȼ�գ�������һ��������ȼ�ϡ��Իش��������⣺
����ͼ��һ����������ȫȼ������CO2��1 mol H2O(l)�����е������仯ͼ������ͼ�е�������������������������_____��

��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��___________________________________��
��������(CH3OCH3)��һ������ȼ�ϣ�Ӧ��ǰ��������1 mol��������ȫȼ������CO2��Һ̬ˮ�ų�1 455 kJ��������1 mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1835 kJ���������������У�����Ͷ����ѵ����ʵ���֮��Ϊ_______��
��2����ѧ�Ҹ�˹������������ܻ�ѧ������һ����ɻ�ּ�����ɣ�����ܹ��̵���ЧӦ����ͬ�ġ������ø�˹���ɿɲ�ijЩ�ر�Ӧ����ЧӦ��
��P4(s������)��5O2(g)===P4O10(s)����H1����2 983.2 kJ��mol��1
��P(s������)��5/4O2(g)===1/4P4O10(s)����H2����738.5 kJ��mol��1
�����ת��Ϊ�����Ȼ�ѧ����ʽΪ_________________________________________����ͬ��״���£������ϵ͵���________�������ȶ��ԱȺ���________(����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ�У���д��ȷ����( )��
A.����ϡ���ᷴӦ��2Fe��6H����2Fe3����3H2��
B.ϡ����������������Һ��Ӧ��Ba2����H����OH-��![]() ��H2O��BaSO4��
��H2O��BaSO4��
C.̼�����ϡ���ᷴӦ��CaCO3��2H����Ca2����CO2����H2O
D.ͭƬ����������Һ��Ӧ��Cu��Ag����Cu2����Ag
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��4NH3+5O2![]() 4NO+6H2O������Ӧ���ʷֱ���v(NH3)��v(O2)��v(NO)��v(H2O)��mol/(L��min)�ݱ�ʾ������ȷ�Ĺ�ϵʽ��
4NO+6H2O������Ӧ���ʷֱ���v(NH3)��v(O2)��v(NO)��v(H2O)��mol/(L��min)�ݱ�ʾ������ȷ�Ĺ�ϵʽ��
A. 4v(NH3)=5v(O2) B. 5v(O2)=6v(H2O) C. 2v(NH3)=3v(H2O) D. 4v(O2)=5v(NO)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com