
����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50 mL 0.25 mol��L��1���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol��L��1NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȡ�
�ش��������⣺
��1������NaOH��Һ����ȷ������__________��
A���ز������������� B���������������� C��һ��Ѹ�ٵ���
��2��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������________��
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β������������ؽ���
��3�������Ϊ�β���ͭ�ʵ�_____________.
��4��ʵ���������±���
������д�±��еĿհף�________��
�¶� ʵ����� | ��ʼ�¶� | ��ֹ�¶�
| �¶Ȳ�ƽ��ֵ | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ�����Ϊ0.55 mol��L��1NaOH��Һ��0.25 mol��L��1������Һ���ܶȶ���1 g��cm��3���кͺ�������Һ�ı�����c��4.18 J��g��1������1�������ϱ����ݼ����к��Ȧ�H��________(ȡС�����һλ)��
���к��Ȳⶨʵ���У����в���һ���ή��ʵ��ȷ�Ե���______��
A���ζ��ܣ���������������������0.01��ȡ���������Һ�����
B��NaOH��Һ�ڵ���С�ձ�ʱ������������
C����С�ձ�������ϴв��ŵ�����ĭ���Ͻ϶�
D������������Һ���¶ȼ���ˮϴ�����������NaOH��Һ���¶�
���𰸡�CDͭ�������õĴ������ܣ���ʹ������ɢ������������ʧ3.4��-56.8kJ/molB
��������
��������NaOH��Һ����С�ձ��У����ּܷ��ε��룬����ᵼ������ɢʧ��Ӱ��ⶨ�����������������������ƻ��ʱ���������¶ȼ��ϵĻ��β������������ؽ�����ʹ������NaOH��Һ��Ͼ��ȣ���4�����ݹ�ʽQ=��H=��Tcm�����㷴Ӧ���ʱ䣬�������Ȼ�ѧ����ʽ����д������д�Ȼ�ѧ����ʽ����5��ʵ�����к��ȵ�ʵ��ֵ����С������ֵ������Ҫԭ��������ɢʧ������ͼʾװ�ò��Ϸ�����ԭ����
��������������������Һʱ������һ��Ѹ�ٵĵ��룬Ŀ���Ǽ���������ɢʧ�����ּܷ��ε�������������Һ������ᵼ������ɢʧ��Ӱ��ⶨ�������ѡC��������ʹ������NaOH��Һ��Ͼ��ȵ���ȷ���������ǣ��������¶ȼ��ϵĻ��β������������ؽ������¶ȼ��Dz����¶ȵģ�����ʹ���¶ȼƽ��裻Ҳ������������ձ���������ܵ���Һ�彦��������ɢʧ��Ӱ��ⶨ����������ܴ�ӲֽƬ�ò��������裬�����������ɢʧ����ѡD��(3)ͭ�������õĴ������ܣ���ʹ������ɢ������������ʧ���ʽ��������ͭ�ʵģ���4����ʵ��1��2��3��4���²�ֱ���3.4�桢5. 1�棨���̫����ȥ����3.3�桢3.5�棬�����¶Ȳ�ƽ��ֵ3.4�棻���������ƹ�������Ӧ������ˮ��0.025mol�����Ԧ�H��![]() ����A���õζ��ܣ���������������������0.01��ȡ���������Һ��������������ȷ�����ʵ��ȷ�ԣ�ѡ��A����B������������Һ����ʱ����������٣���ʹ���к�������ƫС������һ���ή��ʵ��ȷ�ԣ�ѡ��B��ȷ��C����С�ձ��в��ŵ�����ĭ���Ͻ϶࣬����Ч�����ã������ʵ��ȷ�ԣ�ѡ��C����D������HCl��Һ���¶ȼ���ˮϴ���ٲ��������ƣ��������ͼ�֮����Ϊ�кͷ�Ӧ�����µ�������ʧ�����ʵ���ȷ�ȣ�ѡ��D����ѡB��
����A���õζ��ܣ���������������������0.01��ȡ���������Һ��������������ȷ�����ʵ��ȷ�ԣ�ѡ��A����B������������Һ����ʱ����������٣���ʹ���к�������ƫС������һ���ή��ʵ��ȷ�ԣ�ѡ��B��ȷ��C����С�ձ��в��ŵ�����ĭ���Ͻ϶࣬����Ч�����ã������ʵ��ȷ�ԣ�ѡ��C����D������HCl��Һ���¶ȼ���ˮϴ���ٲ��������ƣ��������ͼ�֮����Ϊ�кͷ�Ӧ�����µ�������ʧ�����ʵ���ȷ�ȣ�ѡ��D����ѡB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D�������ʾ�Ϊ����������ɵĿ����Ի����������������ʵ����ӣ����Ӳ����ظ���ϣ��У�
������ | Na+��Al3+��Ba2+��NH4+ |
������ | Cl����OH����CO32����SO42�� |
�ֱ�ȡ�������ʽ���ʵ�飬ʵ����������B��Һ�ֱ���C��D��ϣ����а�ɫ������������A��Һ��ε���C��Һ�У��г������ɣ������μ�A��Һʱ����������ֱ����ȫ��ʧ��A��D���ֹ��������������ɣ���������ʹʪ��ĺ�ɫʯ����Һ��������ʯī�缫���B��Һ���������ϲ���һ���д̼�����ζ������
�ش��������⣺
��1��A�����������Ӻ�C���������ӵİ뾶��С____��______�������ӷ��ţ���B��������������________
��2��C��Һ��___�ԣ�����ԡ����ԡ�������ԭ����__________________
�������ӷ���ʽ���ͣ���D�Ļ�ѧʽ��____________
��3����PtΪ�缫���1L0.1mol/LB��ˮ��Һ������·��ͨ��0.1mol����ʱ��
��Һ��pHΪ_______�����������Һ������䣩�������ĵ缫��ӦʽΪ��_____
��4����������������������ͨ��A��Һ����ǡ����ȫ��Ӧʱ������Һ�и�����
Ũ���ɴ�С������˳��Ϊ__________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�����м������ʣ�A. ![]() B. ����ͼ��� C. ��������춡�� D. CH3CH3��CH3(CH2)8CH3
B. ����ͼ��� C. ��������춡�� D. CH3CH3��CH3(CH2)8CH3
E.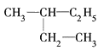 ��
��![]() F.
F.![]() ��
��![]()
���У�������ͬ���칹�����________��������ͬλ�ص���________��������ͬһ�����ʵ���________��
������ͬϵ�����________________��
(2)��֪ij����������Է�������Ϊ72��
�ٸ������ķ���ʽΪ______________��
��д���������������е�ͬ���칹��Ľṹ��ʽ____________________________��
������ͬ���칹���У�����ͬ�����·е���͵���_____________________________(д�ṹ��ʽ)��
(3) ��֪![]() �ı����ϵĶ���ȡ������6�֣���ױ������ϵ�����ȡ������______�֣�
�ı����ϵĶ���ȡ������6�֣���ױ������ϵ�����ȡ������______�֣�
(4)���и��ִ����ܷ�������������_______ ��
A. CH3OH B. CH3CH2OH C. CH3CH(CH3)OH D. CH3CH2C(CH3)2OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£������Ϊ10L�Ĺ̶������з����ķ�Ӧ��N2+3H2![]() 2NH3����Ӧ��������ͼ��ʾ������˵����ȷ����
2NH3����Ӧ��������ͼ��ʾ������˵����ȷ����
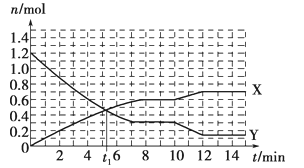
A. t1 minʱ�����淴Ӧ�������
B. X���߱�ʾNH3�����ʵ�����ʱ��仯�Ĺ�ϵ
C. 0��8 min��H2��ƽ����Ӧ����v��H2����0.75 mol��L-1��min-1
D. 10��12 min��N2��ƽ����Ӧ����Ϊv��N2��=0.25mol��L-1��min-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����X��Y��Z��T��U���ֶ�����Ԫ�ء�X��Y��Z��Ԫ�������ڱ��е�λ����ͼ��ʾ,��Ԫ�ص�ԭ������֮����41��X��T�ĵ����ڲ�ͬ�����·�Ӧ,��������T2X(��ɫ����)��T2X2(����ɫ����)���ֻ����U������Z������ȼ��ʱ������ɫ����,�������ˮ��Һ��ʹʯ����Һ��졣
X | |
Y | Z |
(1)��Ԫ�صķ�����X__________,Y__________,Z__________,T__________,U__________
(2)Yԭ�ӵĽṹʾ��ͼΪ__________
(3)�õ���ʽ��ʾY��T��ɵĻ�������γɹ���:__________
(4) YX2��U2Y��Ӧ�Ļ�ѧ����ʽΪ_______________________,������������__________,��������Ԫ����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A. 24 g þ��27 g���У�������ͬ��������
B. ͬ�������������ͳ����У���������ͬ
C. 1 mol��ˮ��1 molˮ�У���������Ϊ2��1
D. 1 mol�����1 mol��ϩ�У���ѧ������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25��ʱ���ܱ�������X��Y��Z��������ij�ʼŨ�Ⱥ�ƽ��Ũ�����±���
���� | X | Y | Z |
��ʼŨ��/mol��L-1 | 0.1 | 0.2 | 0 |
ƽ��Ũ��/mol��L-1 | 0.05 | 0.05 | 0.1 |
����˵��������ǣ�
A. ��Ӧ�ﵽƽ��ʱ��X��ת����Ϊ50�� B. ��Ӧ�ɱ�ʾΪX+3Y![]() 2Z����ƽ�ⳣ��Ϊ1600 C. ����ѹǿʹƽ��������Z�ķ����ƶ���ƽ�ⳣ������ D. �ı��¶ȿ��Ըı�˷�Ӧ��ƽ�ⳣ��
2Z����ƽ�ⳣ��Ϊ1600 C. ����ѹǿʹƽ��������Z�ķ����ƶ���ƽ�ⳣ������ D. �ı��¶ȿ��Ըı�˷�Ӧ��ƽ�ⳣ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʡ�����֮�������µķ�Ӧ��ϵ��

(1)��A�ǵ���ɫ���壬C��D�������C������������Ҫ���ʣ���CҲ����㷺����;��д�����е�������;��____��д���ܵĻ�ѧ����ʽ___________________ ������Cͨ�����Ը��������Һ�У���Һ��ɫ����C����_______�ԡ���ͬ��ͬѹ�£�����C�������������Ϊ1:1ͨ��Ʒ����Һ�У���۲������Ϊ_______�������ӷ���ʽ��ʾ��ԭ��____________________________________________��
(2)��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�������Ҫ�ɷ֣���÷�Ӧ��ѧ����ʽ_______________��д���۵Ļ�ѧ����ʽ��__________________��
(3)��A��̫���ܵ���õĹ�����ϣ�B���������ά��C��DΪ���Ρ�д���ڷ�Ӧ�Ļ�ѧ����ʽ��________________________��C��ˮ��Һ����Ϊ��________��������ܷⱣ�棬��ԭ��Ϊ______________________________���û�ѧ����ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܶ��ʻ���ˮ������ζ���������Ļ�����ͼ����ϩ��Ϊԭ����ȡ���������Ĺ���(���ֲ���ͷ�Ӧ��������ȥ)��
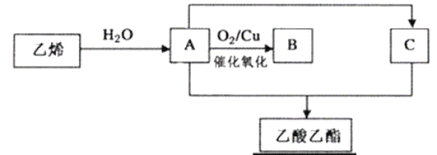
��ش��������⣺
��1��B�Ľṹ��ʽΪ______________��
��2����ϩ��ˮ��Ӧ����A�ķ�Ӧ����Ϊ______________��
��3��A��C��Ӧ�������������Ļ�ѧ����ʽΪ__________���䷴Ӧ����Ϊ__________��
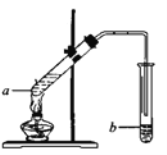
��4��ʵ���ҿ�����ͼװ����ȡ����������
�����Թ�a�мӺ�����Լ�����Ҫ����2��3������Ƭ����������__________��
���Թ�b��ʢ�б���̼������Һ�����ɵ����������ڸ���Һ��_______(��ϡ����¡�)�㣬�÷�Һ�ķ���������ò�Ʒ�������Ҫ������________���Թ�b�еĵ��ܿ�Ӧ��Һ���Ϸ��������뵽Һ�����£���ԭ����______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com