
| A���ڢ� | B���٢� | C���٢� | D���ۢ� |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
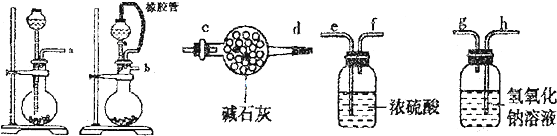
| �ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
| 1 | 1.20 | 16.21 |
| 2 | 3.00 | 18.90 |
| 3 | 4.50 | 19.49 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | C | |
| B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
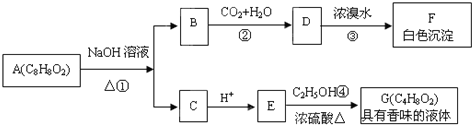
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ca2+��K+��Cl-��CO32- |
| B��Ba2+��Cl-��K+��SO42- |
| C��Fe3+��Cl-��K+��NO3- |
| D��Ag+��NO3-��K+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ֻ����һ��Ԫ�ص�����һ���ǵ��� |
| B����������Һ�ı������������Ƿ��ж�������� |
| C��һ���¶ȡ�ѹǿ�£���������������ʵ����Ķ��پ��� |
| D����ʢ�з�ˮ���ձ��еμ�FeCl3��Һ����ʱ����У��Ʊ�Fe��OH��3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������м�����������õ������Ի����Һ��c��Na+����c��CH3COO-����c��H+����c��OH-�� |
| B��ij��Ԫ�������ʽ��NaHA��Һ�У�c��H+��+c��Na+��=c��OH-��+c��HA-��+c��A2-�� |
| C��1.0 mol?L -1Na2CO3��Һ��c��OH-��=2c��HCO3-��+c��H+��+c��H2CO3�� |
| D�����ʵ���Ũ����ȵģ�NH4��2SO4��NH4HSO4��NH4Cl��Һ��c��NH4+������NH4��2SO4��NH4HSO4��NH4Cl |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com