
����Ŀ����1��һ��SO42- ������������_____����������____������9.6��SO42- �ģ�NH4��2SO4�����У���____����ԭ�ӣ�____mol NH4+��
��2��3mol CH4����_________�����ӣ�______mol ��ԭ�ӣ���CH4��������____�ˡ�
��3��6 mol NH4HCO3�����������____�ˣ����к���ԭ��____mol������ԭ��_____�����������Ӻ������ӹ�__________����
��4������6 mol K+��K2CO3�����ʵ�����_____��K2CO3��������___�����������ܸ�����____����ԭ�ӵ����ʵ�����_____��
��5����7NA����ԭ�ӵ�SO2�У�SO2�����ʵ�����________��SO2��������____����Ԫ�ص�������_______��
��6��H2��CO��������Ϊ1:7ʱ�������ʵ���֮��Ϊ_________��ͬ��ͬѹ�����֮��Ϊ_____��
��7����״����2.24��SO2�У�m��O����__g������Ϊ4�˵�CH4�ڱ���µ������__����
��8�������3.36��NH3�к���Ԫ�ص�������___�ˣ������ӵĸ�����______��
��9����֪m(CO2)=44g��CH4�ķ�����Ϊ1.806��1024����������V(CO2):V(CH4)��_____��
���𰸡�48508��6.02��10220.23��6.02��10231248474301.8��6.02��10241.2��6.02��10243 mol414��9��6.02��10239mol3.5 mol224g 112g2��12��13.222.40.451.5��6.02��10231:3
��������
��1��һ��SO42- ������������16+8��4��48����������48+2��50������9.6��SO42- �ģ�NH4��2SO4����������������ʵ�����9.6g��96g/mol��0.1mol����������淋����ʵ�����0.1mol����ԭ�ӵ����ʵ�����0.1mol��4��2��0.8mol����˺�8��6.02��1022����ԭ�ӣ�����笠������ʵ�����0.2mol��
��2������N��nNA��֪3mol CH4����3��6.02��1023�����ӣ�������ԭ�ӵ����ʵ�����3mol��4��12mol������m��nM��֪��CH4��������3mol��16g/mol��48g��
��3������m��nM��֪6 mol NH4HCO3�����������6mol��79g/mol��474g�����ݻ�ѧʽ��֪���к���ԭ��6mol��5��30mol������ԭ�����ʵ�����6mol��3��18mol������N��nNA��֪��ԭ�Ӹ�����1.8��6.02��1024����̼�������笠���̼�����������ɣ���˺������Ӻ������ӵ������ʵ�����6mol��2��12mol��������1.2��6.02��1024����
��4������6 mol K+��K2CO3�����ʵ�����6mol��2��3mol��K2CO3��������3mol��138g/mol��414g��̼����ɼ����Ӻ�̼���������ɣ���˺������Ӻ������ӵ������ʵ�����3mol��3��9mol��������9��6.02��1023�������ݻ�ѧʽ��֪��ԭ�ӵ����ʵ�����3mol��3��9mol��
��5����7NA����ԭ�ӵ�SO2����ԭ�ӵ����ʵ�����7mol����SO2�����ʵ�����7mol��2��3.5mol������m��nM��֪SO2��������3.5mol��64g/mol��224g����Ԫ�ص�������7mol��16g/mol��112g��
��6��H2��CO��������Ϊ1:7������m��nM��֪�����ʵ���֮��Ϊ![]() �����ݰ����ӵ����ɿ�֪��ͬ���������֮�������ʵ���֮�ȣ���ͬ��ͬѹ�����֮��Ϊ2��1��
�����ݰ����ӵ����ɿ�֪��ͬ���������֮�������ʵ���֮�ȣ���ͬ��ͬѹ�����֮��Ϊ2��1��
��7����״����2.24LSO2�����ʵ�����2.24L��22.4L/mol��0.1mol������ԭ�ӵ����ʵ�����0.2mol������m��O����0.2mol��16g/mol��3.2g������ԭ��Ϊ4�˵�CH4��������ԭ�ӵ����ʵ�����4mol�����������ʵ�����4mol��4��1mol���ڱ���µ������22.4L��
��8�������3.36��NH3�����ʵ�����3.36L��22.4L/mol��0.15mol����ԭ�ӵ����ʵ�����0.15mol��3��0.45mol�������к���Ԫ�ص�������0.45�ˡ�������10�����������ӵ����ʵ�����1.5mol��������1.5��6.02��1023��
��9����֪m(CO2)=44g���������̼�����ʵ�����44g��44g/mol��1mol��CH4�ķ�����Ϊ1.806��1024�������������ʵ�����![]() ���������ͬ��������������֮�������ʵ���֮�ȿ�֪�����V(CO2):V(CH4)��1:3��
���������ͬ��������������֮�������ʵ���֮�ȿ�֪�����V(CO2):V(CH4)��1:3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ1��ϸ���л����ﺬ��������ͼ��ͼ2���л��Ե�ϸ����Ԫ�غ���������ͼ������˵������ȷ���ǣ� ��

A. ��ͼ1��ʾϸ�����أ���A��B������������H2O��������
B. ��ͼ2��ʾ�������ϸ����Ԫ�غ�������a��b��c������O��C��H
C. �ؿ����ϸ���к�������Ԫ�ض���a�����˵������������������ͳһ��
D. ��ͼ1��ʾϸ����ȫ��ˮ����ĺ�������A��������ж����ԣ����к���Ԫ��ΪC��H��O��N
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������β����CO��NOx�Լ�ȼú�����е�SO2���Ǵ�����Ⱦ������ǵ�����������Ҫ���塣
��1��������ԭ������NOx��ת�����£�
![]()
�ٷ�ӦIΪNO +O3===NO2+O2�����ɱ�״����11.2 L O2ʱ��ת�Ƶ��ӵ����ʵ�����__________mol
�ڷ�Ӧ���У���n( NO2)��n[CO(NH2)2]=3��2ʱ�����������뻹ԭ�����������Ϊ___________
��2������SO2��NO�����Na2S2O4��NH4NO3��Ʒ������ͼ���£�CeΪ��Ԫ�أ���װ��I�з�����Ӧ�����ӷ���ʽΪ____________________________________

��3��װ�����У�����������NO��Ce4+�����IJ�����Ҫ��NO3����NO2������д�����ɵ����ʵ�����NO3����NO2��ʱ�����ӷ���ʽ____________________________________
��4��װ����������֮һ�������ӽ���Ĥ���۵��ʹ��Ce4+����������ʱ���ɵ�Ce4+�ڵ��۵�_____________��������������������������ͬʱ����һ������S2O42һ�ĵ缫��ӦʽΪ_________________________________
��5��ȡ����װ�������õIJ�Ʒ����ˮ����Һ������Ũ���ɴ�С��˳��Ϊ_________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����G=��H��T��S������G��0����Ӧ���Է���������G��0��Ӧ�����Է�������ij��Ӧ2AB(g�� ![]() C(g����3D(g���ڸ���ʱ���Է����У��ڵ����²����Է����У���÷�Ӧ������Ӧ����H����SӦΪ
C(g����3D(g���ڸ���ʱ���Է����У��ڵ����²����Է����У���÷�Ӧ������Ӧ����H����SӦΪ
A. ��H��0����S��0 B. ��H��0����S��0
C. ��H��0����S��0 D. ��H��0�� ��S��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.8 mol I2(g)��1.2 mol H2(g)����ij1L�ܱ�����������һ���¶��·�����Ӧ��I2(g)��H2(g) ![]() 2HI(g) ��H <0���ﵽƽ�⡣HI�����������ʱ��ı仯�������ʾ��
2HI(g) ��H <0���ﵽƽ�⡣HI�����������ʱ��ı仯�������ʾ��
HI������� | 1min | 2min | 3min | 4min | 5min | 6min | 7min |
����I | 26% | 42% | 52% | 57% | 60% | 60% | 60% |
����II | 20% | 33% | 43% | 52% | 57% | 65% | 65% |
����˵������ȷ����
A. ������I�£��÷�Ӧ��ƽ�ⳣ��K=10
B. ������I�£��ӿ�ʼ��Ӧ��5min����H2��ʾ�ķ�Ӧ����Ϊ0.10 mol/(L��min)
C. ������II�£�����ƽ��ʱ�� I2(g)��ת����Ϊ81.25%
D. ������I��ȣ�Ϊ�ﵽ����II�����ݣ����ܸı�������ǽ���ѹǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з���������ԭ�Ӷ����������Ϊ8���ӽṹ���ǣ� ��
A.BF3B.H2OC.CCl4D.PCl5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�� 25��ʱ�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L������������Һ��
�� Na2CO3��Һ �� NaHCO3��Һ �� NaF��Һ ��NaClO��Һ�����������ж�pH�ɴ�С��˳����_________________________��
��2�����ˮƿˮ���к��е�CaSO4�ķ���������ˮƿ�е�ˮ����̼���Ʊ�����Һ���ݺ��ˮ��ϴ���ټ������ἴ�ɣ���д��������Ӧ�Ļ�ѧ����ʽ��________________��_____________ ��
��3��Na2CO3��Һ�Լ�������ΪCO32-ˮ���Ե�ʣ�����Ƽ�ʵ����ʵ֤��֮____________________��
��4������ƽ�ⳣ������K��ʾ)��һ��ƽ�ⳣ��������ƽ�ⳣ���Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��
��ѧʽ | HF | H2CO3 | HClO |
����ƽ�ⳣ����K�� | 7.2��10-4 | K1=4.4��10-7 K2=4.7��10-11 | 3.0��10-8 |
�����ĵ��뷽��ʽΪ____________________�����������ƽ�ⳣ���ı���ʽΪ_______________��
��5��25��ʱ��pH=3��HF��Һ�У�δ����ķ��������ʵ���Ũ��Ϊ_______________________mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����豶���������к��в��ӣ���������Ŀǰ�в����˹��ϳɵĴ���Ȼ����ܡ���Ч�ܵĿ������������ɻ�������������ûʳ�Ӷ�����(EGC)�Ľṹ��ͼ��ʾ������EGC������˵������ȷ����
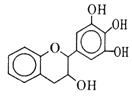
A. ��FeCl3��Һ��������ɫ��Ӧ
B. 1 mol EGC��4 mol NaOHǡ����ȫ��Ӧ
C. ����������Ӧ��ȡ����Ӧ���ѷ����ӳɷ�Ӧ
D. ���������е�ԭ�ӹ�ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���������( )
A. �Ҵ���ʹ���Ը��������Һ��ɫ������������CO2��H2O
B. ![]() ��C4Hl0�Ķ��ȴ������Ŀ��ͬ�����������칹��
��C4Hl0�Ķ��ȴ������Ŀ��ͬ�����������칹��
C. ��ϩ��ʹ��ˮ��ɫ����ʹ����KMnO4��Һ��ɫ�����Ƿ�����Ӧ�����Ͳ�ͬ
D. ֲ����ͨ���⻯���Ա��֬��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com