
����Ŀ��һ���¶��£���ij�ܱ������з�����Ӧ��2A(g)![]() B(g)��C(s)����H>0����0��15 s��c(A)��0.1 mol��L��1����0.07 mol��L��1��������˵����ȷ����(����)
B(g)��C(s)����H>0����0��15 s��c(A)��0.1 mol��L��1����0.07 mol��L��1��������˵����ȷ����(����)
A. 0��15 s����C��ʾ��ƽ����Ӧ����Ϊv(C)��0.001 mol��L��1��s��1
B. 0��15 s��v(B)��0.002 mol��L��1��s��1
C. �����¶�����Ӧ���ʼӿ죬�淴Ӧ���ʼ���
D. ��С��Ӧ��ϵ���������ѧ��Ӧ���ʼӿ�
���𰸡�D
��������
A��C��״̬Ϊ���壬Ũ��Ϊ���������ܱ�ʾ��ѧ��Ӧ���ʣ���A����
B�����ݻ�ѧ��Ӧ������ѧ����ʽ��v(A)=(0.1��0.07)mol/L��15s=0.002mol/(L��s)�����ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ�v(B)=v(A)/2=0.002mol/(L��s)��2=0.001mol/(L��s)����B����
C�������¶ȣ����淴Ӧ���ʶ��ӿ죬��C����
D����С��Ӧ��ϵ�������ѹǿ����ѧ��Ӧ���ʼӿ죬��D��ȷ����ѡD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�߷��ӻ�����I���Ṥҵ��������Ҫԭ�ϣ�����һ�������ϳ�·�����£�

��֪��
�ش��������⣺
��1��AΪ��������A�Ļ�ѧ������______��
��2��G��H��H��I�ķ�Ӧ���ͷֱ���______��______��
��3����G�ṹΪ 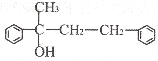
����д��D�Ľṹ��ʽ______��
��2��B����C�Ļ�ѧ����ʽΪ__��
��4��C�ж���ͬ���칹�壬�������ڷ����ͬ���칹����_____�֣�д�����о�������˴Ź��������һ�ֽṹ��ʽ_____��
��5���ο������ϳ�·����Ϣ��д����CH3��CH=CH2��CH3MgBrΪԭ��(����������ѡ)�ϳ� �ĺϳ�·��ͼ��_____
�ĺϳ�·��ͼ��_____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ݱ�����������M�Է��ѻ�ù���нϺõ��־����ԣ���ϳ�·������ͼ��ʾ��

��֪��
�ش��������⣺
��1��������C�еĺ�������������Ϊ_________��A��B�ķ�Ӧ����Ϊ__________��
��2��A������Ϊ____________
��3��д��D�Ľṹ��ʽ��_____________________________��
��4��д����Ӧ�ڵĻ�ѧ����ʽ��____________________________________________��
��5��������C��������������ͬ���칹����_______�֣�д������һ����5�ֲ�ͬ������ԭ�ӵ�ͬ���칹��Ľṹ��ʽ��_______________��
�ٺ������ṹ�����ڼ��������·���ˮ�⣻
������FeCl3������ɫ��Ӧ��
���ܷ���������Ӧ��
��6����֪CH3CH2CN![]() CH3CH2COOH������
CH3CH2COOH������ ��CH2=CHCN���Ҵ�Ϊԭ�Ϻϳɻ�����
��CH2=CHCN���Ҵ�Ϊԭ�Ϻϳɻ�����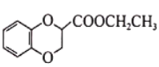 ��д���Ʊ��ĺϳ�·������ͼ�����Լ����ã�____________________
��д���Ʊ��ĺϳ�·������ͼ�����Լ����ã�____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ijͬѧ�����һ��ȼ�ϵ�ز�̽���ȼҵԭ���ʹ�ͭ����������п�����������ʣ��ľ���ԭ����������װ����XΪ�����ӽ���Ĥ���ش��������⣺

(1)����ȼ�ϵ�ظ����ĵ缫��ӦʽΪ_________��
(2)��װ����������ͨ��XĤ��________ �����Fe����C�����ƶ�����װ���д�ͭΪ_______�缫���A����B����
(3)���ڱ���£���2.24L�������뷴Ӧ������װ�����Ҳ���Һ________������ӡ����١���______g����װ����CuSO4Ũ��__________������ӡ��������١� ���䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��J�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮�����ϵ����ͼ��ʾ(���ַ�Ӧ�������û���г�)������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�

��ش��������⣺
(1) NaOH�ĵ���ʽΪ__________________��
(2) G�Ļ�ѧʽΪ______________________��
(3) д����Ӧ�ܵ����ӷ���ʽ��________________________________________________��
(4) д����Ӧ�Ļ�ѧ����ʽ��____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ں����ܱ�������ͨ��X��������Ӧ��2X(g)![]() Y(g)���¶�T1��T2��X�����ʵ���Ũ��c(X)��ʱ��t�仯��������ͼ��ʾ������������ȷ����
Y(g)���¶�T1��T2��X�����ʵ���Ũ��c(X)��ʱ��t�仯��������ͼ��ʾ������������ȷ����

A. �÷�Ӧ���е�M��ų����������ڽ��е�W��ų�������
B. T2�£���0��t1ʱ���ڣ���(Y)��a-b/t1����mol/(L��min)
C. M�������Ӧ����������������N����淴Ӧ���������棩
D. M��ʱ�ټ���һ������X��ƽ���X��ת���ʼ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��KI���ڷ����Լ����й������ҩ��ʳƷ���Ӽ��ȡ�
�Ʊ�ԭ�����£���Ӧ�� 3I2+6 KOH =a KIO3+5KI+3H2O
��Ӧ�� 3H2S+KIO3=3S��+KI+3H2O

��������ʵ����̣���ش��й����⡣
��1�����շ������з����Ļ�ѧ����ʽΪ______________________���ø�װ�û������Ʊ�___________(��һ�����廯ѧʽ)��
��2���ر����շ�������������Һ©���Ļ���������30%��KOH��Һ�����۲쵽______________________(������)��ֹͣ����KOH��Һ��Ȼ��______________________(�����)����KIO3���Һ��NaOH��Һ�������ʽӽ���ͬʱֹͣͨ����
��3������������Һ������KI���Һˮԡ���ȣ���Ŀ����______________________��
��4����KI���Һ�����ձ�������̼�ᱵ���ڹ������й��ˣ����˵õ��ij����г����й���̼�ᱵ�⣬�������ᱵ��___________�����м���̼�ᱵ��������______________________���ϲ���Һ��ϴҺ�������������ᾧ���˳�������ó�Ʒ��
��5������õ�3.2g���ʣ����������Ƶõ�KIΪ___________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������İ�ˮ�ӵ�����ͭ��Һ�У���Һ���ձ������ɫ�����������Ҵ�����������ɫ�ľ���[Cu(NH3)4]SO4��H2O��
(1)Cu2+�۵����Ų�ʽΪ___________��[Cu(NH3)4]SO4��H2O�У�1mol[Cu(NH3)4]2+���ЦҼ�����ĿΪ___________��
(2)SO42����Sԭ�ӹ���ӻ�����Ϊ___________��H3O+���ι���Ϊ___________��
(3)NH3��������H2O�У����ܵ�ԭ��Ϊ___________��
(4)N��O��S��һ�������ɴ�С��˳��Ϊ___________��

(5)Cu��F�γɵĻ�����ľ����ṹ��ͼ��ʾ���������ܶ�Ϊag��cm��3����Cu��F�������Ϊ___________pm(��NA��ʾ�����ӵ�������ֵ���г��������ʽ�����û���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ����٤�����������и�ѡ������ȷ���ǣ� ��
A.1mol/L Na2CO3��Һ�е�Na+��ĿΪ2NA
B.��������NO2��N2O4��ǰ��ԭ����С
C.1L 0.1mol/L NaHCO3��Һ�к���ԭ����0.3NA
D.���³�ѹ�£�0.2mol CO2���������4.48L
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com