
����Ŀ����3molA�����1.5molB������3L�������л�ϲ���һ�������������·�Ӧ�� 2A(g)+B(g) ![]() 2C(g)����2s����C��Ũ��Ϊ0.3mol/L�������м�����ȷ���ǣ�
2C(g)����2s����C��Ũ��Ϊ0.3mol/L�������м�����ȷ���ǣ�
����A��ʾ��ƽ����Ӧ����Ϊ0.3mol/��L��s����
��2sʱ����A��Ũ��Ϊ0.7mol/L
����B��ʾ��ƽ����Ӧ����Ϊ0.15mol/��L��s��
��2sʱ����B��ת����Ϊ30��
A.�ڢ�B.�٢�C.�ڢ�D.�٢�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CS(NH2)2(���壬��ɫ���й���ľ��壬����ˮ��20��ʱ�ܽ��Ϊ13.6g����150 ��ʱת��� NH4SCN)����������ҩ�Ⱦ�ϡ���������ĸ�ѡ���ȵ�ԭ�ϡ�ij��ѧʵ��С��ͬѧ��Ca(HS)2��CaCN2(ʯ�ҵ�)�ϳ����岢̽��������
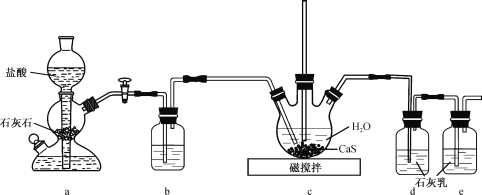
(1)�Ʊ�Ca(HS)2��Һ������װ����ͼ(��֪���ԣ�H2CO3��H2S)��
��װ��a�з�Ӧ�����IJ���Ϊ ______________________________________��װ��b��ʢ�ŵ��Լ���__________________ ��
��װ��c�еij�ֱ���ܵ�������____________________________________��������ƿ��ͨ��CO2 ���ܹ�����ԭ���� ______________________________ ��
(2)�Ʊ����壺��CaCN2��Ca(HS)2��Һ��ϣ�������80��ʱ���ɺϳ����壬ͬʱ����һ�ֳ����ļ���ʵļ��ȷ�ʽ��___________________________���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________________________________________��
(3)̽����������ʣ�
��ȡ������������ˮ�����ȣ���֤��NH4SCN���ɣ����õ��Լ���_____________(�ѧʽ����ͬ)
����ʢ������������Թ��м���NaOH��Һ����NH3�ų������������ķ���Ϊ __________________________________________________��
�ۿ�������KMnO4��Һ�ζ����壬��֪MnO4- ����ԭΪMn2����CS(NH2)2������ΪCO2��N2��SO42�� ���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ҫ��MoS2��������Ca��Si��Cu��Zn��Fe��Ԫ�ء����û�����Ʊ�������淋���һ�����������������ͼ��ʾ��
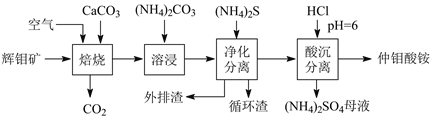
�ش��������⣺
��1�������¶�Ϊ400�棬MoS2ת��ΪCaMoO4��CaSO4����Ӧ��ÿĦMoS2ת�Ƶĵ�����Ϊ_________��������泥�������泥��е���������Mo7O24n-����n��_______��
��2����ͳ��������650���£�ʹMoS2ֱ��������е�O2��Ӧ����MoO3��SO2��ͼʾ����������ռ���CaCO3���ŵ���______________��
��3���ܽ�ʱ��CaMoO4�������ֽⷴӦ�Ļ�ѧ����ʽ��___________��ѭ��������Ҫ�ɷ���CaCO3������������Ҫ��________��Cu��Zn��Fe�����
��4����֪��������Ksp(CaCO3)��2.8��10-9��Ksp(CaSO4)��9.1��10-6����(NH4)2SO4ĸҺ������ѭ������CaCO3��������ʹ�����ת��Ϊ̼��泥������ܽ�ѭ��ʹ�ã���ԭ����_______��
��5���������ֽ��⾫��ʱ���ö��Ե缫����⾫���NaCl�Ļ�Ͻ�Һ��������Ĥ��������ҺpH��9������������Ϊ________����Ͻ�Һ�У���������ת�����ɵ�NaClO����MoS2����MoO42-��SO42-�����ӷ���ʽ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������������ȷ���ǣ� ��
A.1mol NH4+�к��еĵ�����Ϊ11 NA
B.0.1mol��L-1��Ba(OH)2��Һ�к��е�OH- ��ĿΪ0.2NA
C.0.1NA���ȷ�������1Lˮ�У�������Һ��c(C1-)=0.1mol��L-1
D.1mol NH3����ˮ�����1L��Һ�����ð�ˮ�����ʵ���Ũ��Ϊ1mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���һ�ֳ�����ȼ�ϣ�Ҳ����Ҫ�Ļ�������ԭ�ϡ��ش��������⣺
��1�����ü״����ఱ�������Ƶö��װ���
��֪��2CH3OH(g)��3O2(g) 2CO2(g)��4H2O(g) H1��-3122kJ/mol
4NH3(g)��3O2(g) 2N2(g)��6H2O(g) H2��-472 kJ/mol
4(CH3)2NH(g)��15O2(g) 8CO2(g)��14H2O(g)��2N2(g) H3��-7492 kJ/mol
���Ʊ����װ���Ӧ2CH3OH(g)��NH3(g) (CH3)2NH(g)��2H2O(g)��H��_____ kJ/mol��
��2��һ�������£��״����ఱ����ԭ������c(CH3OH):c(NH3)�ֱ�Ϊ1:1��2:1��3:1ʱ��NH3��ƽ��ת�������¶ȱ仯�Ĺ�ϵ��ͼ��
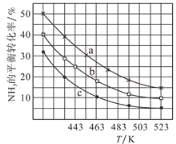
�ٴ���ԭ������c(CH3OH):c(NH3)��1:1��������________��
��һ���¶��£�����ԭ������c(CH3OH):c(NH3)��3:1��������NH3ƽ��ת���ʵĴ�ʩ��_______��
���¶�Ϊ443Kʱ����c(CH3OH):c(NH3)��2:1Ͷ�ϣ���NH3����ʼŨ��Ϊ2mol/L����Ӧ�ﵽƽ��ʱ��(CH3)2NH���������Ϊ_______�����¶��µĻ�ѧƽ�ⳣ��Ϊ________ ��
��3���״���ͨ���绯ѧ�����ɼ���ֱ���Ƶã�װ������ͼ��ʾ���������CH3OH�Ĺ��̷�Ϊ3����

��ͨ��ʱ����������ת��Ϊ���Ե�ԭ���ȣ�Cl������
��Cl���������ڵ缫�ϵ�CH4��Ӧ����HCl��CH3Cl��
���ڼ��Ե��Һ�У�CH3Clת��ΪĿ�����CH3OH��
����ٵĵ缫��ӦʽΪ__________������۵����ӷ���ʽΪ__________��ά�ֵ���ǿ��Ϊ1.5A��װ�ù���2Сʱ�������Ͽ��Ƶ�CH3OH������Ϊ________g������֪F��96500C/mol�������������ܽ�����أ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������й���������Ի�ѧ��Ӧ���ʺͻ�ѧƽ��Ӱ���ͼ������ͼ���ʵ����۱������ȷ����
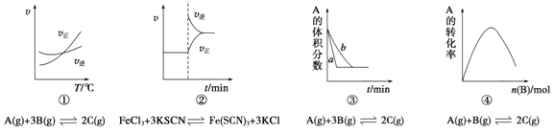
A. ������������һ��ʱ����Ӧ�������¶ȱ仯��ͼ��������Ӧ��H��0
B. ������ƽ����ϵ����Һ����������KCl�����ѧ��Ӧ������ʱ��仯��ͼ��
C. ���������������������£�����ƽ����̵�ͼ��a��ʹ�ô���ʱ������
D. ����һ�������£�����һ����A�ĺ����ܱ�����������B����ƽ��ʱA��ת���ʵ�ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾת����ϵ���漰�������ʾ�������Ԫ����ɣ����ֲ�������ȥ��������C��DΪ���ʣ�A��B��E��F��GΪ�������C��D�ķ�Ӧ�⣬������Ӧ������Һ�н��С�����д���пհס�
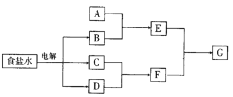
��1�� ��A�dz����Ľ���������������F����ʱ��GΪ�������Σ���A�Ļ�ѧʽΪ_________��_________��
��2�� ��A��һ�ֳ������������F�������GΪͬһ�����������ʣ���A��������________������������_______��A����;��_________��
��3�� ��A��һ�־���Ư���Ե���̬�����A��___����A��һ����ζ����̬�����A�ĵ���ʽΪ______��
��4�� ��AΪ�л���������A��GΪͬһ���ʣ���д������A����ͬ������ʣ��Ľṹ��ʽ��_____��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO2��һ���������壬����������滷���������Ӱ�죬ά�ִ�����CO2��ƽ�����̬��������������Ҫ���塣
I��������CH4��CO2�Ʊ��ϳ���(CO��H2)����ijһ�����ܱ�������CH4��CO2�ķ�ѹ�ֱ�Ϊ15kPa��20kPa������Ni/��-A12O3������������1123Kʹ�䷢����Ӧ��CH4(g)+CO2(g)=2CO(g)+2H2(g)
��1���о�����CO����������![]() ��ijʱ�̲��p(H2)=10kPa�����ʱ��v(CH4)=___________k��Pas-1��
��ijʱ�̲��p(H2)=10kPa�����ʱ��v(CH4)=___________k��Pas-1��
��2���ﵽƽ����CO�IJ���Ϊ50������÷�Ӧ��ƽ�ⳣ��Kp=_________��
��3����ѧ������Ʊ����ϳ�����Ӧ���̷�������
��Ӧ�٣�CH4(g)=C(ads)+2H2(g)(����Ӧ)
��Ӧ�ڣ�C(ads)+CO2(g)=2CO(g)(�췴Ӧ)
������Ӧ��C(ads)Ϊ�����Ի���̿����Ӧ���̵������仯��ͼ��ʾ��
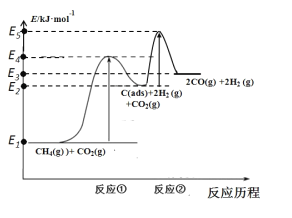
CH4��CO2�Ʊ��ϳ������Ȼ�ѧ����ʽΪ__________________�������仯ͼ�У�E5+E1_________E4+E2�����������������������
II��CO2�������Ƽ״�5MPaʱ����ij�ܱ������а�Ͷ�ϱ�n(H2):n(CO2)=3:1����H2��CO2���������·�Ӧ��
i. ![]() ��
��![]()
ii.. ![]()
iii. ![]()
��Ӧ�ﵽƽ��ʱ����ø���ֵ����ʵ����������¶ȱ仯����������ͼ��ʾ��
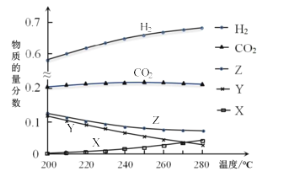
��4������ϵ��CO2�����ʵ����������¶ȵ�Ӱ�첻��ԭ����_______________________��
�����д�ʩ�У�����״�ƽ����ʵ���________(����)��
A.��������CO B.����ѹǿC.ѭ������ԭ����D.�����¶�
������ͼX��Y�ֱ����_________��________(�ѧʽ)��
III������ͭ�������1,10-phenanthroline-Cu�������CO2��ԭ�Ʊ�̼��ȼ��(����CO�����������)�Ǽ���CO2�ڴ������ۻ���ʵ�ֿ�������Դ��Ч���õĹؼ��ֶ�֮����װ��ԭ����ͼ��ʾ��
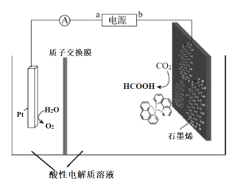
��5���ٵ�ع��������У�ͼ��Pt�缫������Һ��pH_______(����С��)�������ĵ缫��ӦʽΪ________________��
��ÿת��2mol���ӣ���������Һ��������______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ��������ֱ���Na2CO3��Һ��AgNO3��Һ��BaCl2 ��Һ������������ɫ��Һ�е�һ�֣��ֽ�������ͼ��ʾ��ʵ�顣

��1��ͨ������ʵ���жϳ������ʣ���___����___����___����____��д�������ҷ�Ӧ�����ӷ���ʽ��___��
��2��ʵ������һƿ��ǩ������ͼ��ʾ���Ĺ����Լ���ijͬѧӦ�ã�1���е�BaCl2��Һ���飬ȡ�Լ�ƿ�еĹ����������Թ��У�����������ˮ�ܽ⣬��������Һ�м������BaCl2��Һ���õ���ɫ�������ɴˣ���ͬѧ�ƶ���ƿ�Լ��������ơ�����Ϊ���Ľ����Ƿ���ȷ? _____�����ȷ������ȷ����������ȷ����д����Ӧ��Ӧ�����ӷ���ʽ__________��������ȷ, ��˵�����ܵĽ��ۣ�_______�������ʵĻ�ѧʽ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com