
°ĺŐ‚ńŅ°Ņ°ĺĽĮ—ß°™—°–ř3£ļőÔ÷ ĹŠĻĻ”Ž–‘÷ °Ņļ¨Ķ™ĽĮļŌőÔ‘ŕ…ķĽÓ÷–”¶”√ ģ∑÷Ļ„∑ļ°£…Ů÷›∑…ī¨Õ‚Ņ« Ļ”√ŃňĶ™ĽĮĻŤ–¬–ÕŐ’ī…ĹŠĻĻ≤ńŃŌ£¨ł√≤ńŃŌ”≤∂»īů°ĘńÕń•ňū°£Ņ…”√ Į”Ę”ŽĹĻŐŅ‘ŕ1400~1450°śĶńĶ™∆Ý∆Ý∑’Ō¬ļŌ≥…Ķ™ĽĮĻŤ£¨Õ¨ Ī…ķ≥…“Ľ÷÷”ŽĶ™∆ÝĹŠĻĻŌŗň∆Ķń∆ÝŐ¨∑÷◊”°£
£®1£©–ī≥Ų…Ō Ų∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ____________________________________°£∑ī”¶‘≠ŃŌ÷–…śľįĶń‘™ňōĶÁłļ–‘”…īůĶĹ–°ĶńŇŇŃ–ň≥–Úő™________________________________°£
£®2£©ĽýŐ¨Ķ™‘≠◊”÷–Ķń‘≠◊”ĻžĶņ–ő◊ī”– _______________÷÷°£
£®3£©ń≥Õ¨—߼≠≥ŲŃňĻŤ‘≠◊”ĽýŐ¨ĶńļňÕ‚ĶÁ◊”ŇŇ≤ľÕľ»ÁŌ¬Õľ£¨ł√ĶÁ◊”ŇŇ≤ľő•Ī≥Ńň ‘≠ņŪ°£
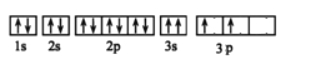
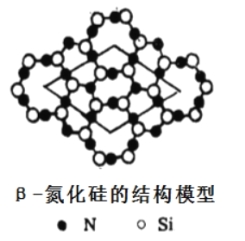
£®4£©Ķ™ĽĮĻŤ”–∂ŗ÷÷–ÕŐŚ£¨∆š÷–¶¬-Ķ™ĽĮĻŤ≤„◊īĹŠĻĻń£–Õ»ÁÕľ£¨“‘Õľ÷–ňý ĺĶń∆Ĺ––ňńĪŖ–őő™ĽýĪĺ÷ōłīĶ•‘™őřŌř…ž’Ļ£¨‘Úł√ĽýĪĺĶ•‘™÷–ļ¨Ķ™‘≠◊”______łŲ£¨ĻŤ‘≠◊”______łŲ°£
£®5£©ļ¨Ķ™Ķń”–ĽķĽĮļŌőÔįĪĽý““ňŠH2NCH2COOH÷–ĶńŐľ ‘≠◊”‘”ĽĮ∑Ĺ Ĺ”–____________£¨¶“”Ž¶–ľŁĶńłŲ żĪ»ő™_______________°£
£®6£©įĪ∑÷◊” «“Ľ÷÷≥£ľŻĶńŇšŐŚ°£Cu2+ņŽ◊”‘ŕňģ»‹“ļ÷–“‘ [Cu £®H2O£©4]2+–ő Ĺīś‘ŕ£¨ŌÚļ¨Cu 2+ņŽ◊”Ķń»‹“ļ÷–ľ”»Ž◊„ŃŅįĪňģ£¨Ņ……ķ≥…łŁő»∂®Ķń[Cu£®NH3£©4]2+ņŽ◊”£¨∆š‘≠“Ú « °£ń≥ŇšļŌőÔĶńĽĮ—ß Ĺő™ CoCl3°§4NH3£¨ ńŕĹÁő™’żįň√śŐŚĻĻ–ÕŇšņŽ◊”°£0.1 molł√ĽĮļŌőÔ»‹”ŕňģ÷–£¨ľ”»ŽĻżŃŅ AgNO3£¨”–14.35g į◊…ę≥ŃĶŪ…ķ≥…°£‘ÚňŁĶń÷––ńņŽ◊”ľŘĶÁ◊”ŇŇ≤ľ Ĺő™____________£¨É»ĹÁŅ…ń‹ĶńĹŠĻĻ”–__________÷÷°£
°ĺīūįł°Ņ£®1£©3SiO2+6C+2N2![]() Si3N4+6CO O>N>C> Si £®2£© 2 £®3£©Ň›ņŻ≤ĽŌŗ»›
Si3N4+6CO O>N>C> Si £®2£© 2 £®3£©Ň›ņŻ≤ĽŌŗ»›
£®4£©8 6 £®5£©sp2,sp3 9:1 £®6£© N‘™ňōĶÁłļ–‘łŁ–°£¨łŁ“◊łÝ≥ŲĻ¬∂‘ĶÁ◊”–ő≥…ŇšőĽľŁ£Ľ 3d6 2
°ĺĹ‚őŲ°Ņ
‘Ő‚∑÷őŲ£ļ£®1£©”√ Į”Ę”ŽĹĻŐŅ‘ŕ1400°ę1450°śĶńĶ™∆Ý∆Ý∑’Ō¬ļŌ≥…Ķ™ĽĮĻŤ£¨Õ¨ Ī…ķ≥…“Ľ÷÷”ŽĶ™∆ÝĹŠĻĻŌŗň∆Ķń∆ÝŐ¨∑÷◊”£¨łý囑≠◊” ōļ„“‘ľįĶ™∆ÝĶńĹŠĻĻŅ…÷™ł√∆ÝŐ¨∑÷◊””¶ł√ «CO£¨ňý“‘…Ō Ų∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™3SiO2+6C+2N2![]() Si3N4+6CO°£∑ī”¶‘≠ŃŌ÷–…śľįĶń‘™ňō”–C°ĘN°ĘO°ĘSi£¨∑«Ĺū Ű–‘‘Ĺ«ŅĶÁłļ–‘‘Ĺīů£¨“ÚīňĶÁłļ–‘”…īůĶĹ–°ĶńŇŇŃ–ň≥–Úő™O>N>C>Si°£
Si3N4+6CO°£∑ī”¶‘≠ŃŌ÷–…śľįĶń‘™ňō”–C°ĘN°ĘO°ĘSi£¨∑«Ĺū Ű–‘‘Ĺ«ŅĶÁłļ–‘‘Ĺīů£¨“ÚīňĶÁłļ–‘”…īůĶĹ–°ĶńŇŇŃ–ň≥–Úő™O>N>C>Si°£
£®2£©ĽýŐ¨Ķ™‘≠◊”ļňÕ‚ĶÁ◊”ŇŇ≤ľő™1s22s22p3£¨ňý“‘‘≠◊”÷–Ķń‘≠◊”ĻžĶņ–ő◊ī”–2÷÷°£
£®3£©Õ¨“ĽłŲĻžĶņ÷–ĶÁ◊”Ķń‘ň∂Į∑ĹŌÚŌŗÕ¨£¨ňý“‘ł√ĶÁ◊”ŇŇ≤ľő•Ī≥ŃňŇ›ņŻ≤ĽŌŗ»›‘≠ņŪ°£
£®4£©łýĺ›ń£–ÕŅ…÷™Si‘≠◊”»ę≤Ņ‘ŕ∆Ĺ––ňńĪŖ–ő÷–£¨Ļ≤ľ∆ «6łŲ£ĽN‘≠◊”łŲ ż «4£ę8°Ń1/2£Ĺ8°£
£®5£©ļ¨Ķ™Ķń”–ĽķĽĮļŌőÔįĪĽý““ňŠH2NCH2COOH÷–Ű»ĽýĶńŐľ‘≠◊”‘”ĽĮ∑Ĺ Ĺő™sp2‘”ĽĮ£¨Ī•ļÕŐľ‘≠◊”ő™sp3‘”ĽĮ£ĽĶ•ľŁ∂ľ «¶“ľŁ£¨ň꾣ļ¨”–“ĽłŲ¶“ľŁļÕ1łŲ¶–ľŁ£¨“Úīň¶“”Ž¶–ľŁĶńłŲ żĪ»ő™9:1°£
£®6£©”…”ŕN‘™ňōĶÁłļ–‘łŁ–°£¨łŁ“◊łÝ≥ŲĻ¬∂‘ĶÁ◊”–ő≥…ŇšőĽľŁ£¨ňý“‘Ņ……ķ≥…łŁő»∂®Ķń[Cu£®NH3£©4]2+ņŽ◊”£Ľ
0.1 molł√ĽĮļŌőÔ»‹”ŕňģ÷–£¨ľ”»ŽĻżŃŅ AgNO3£¨”–14.35gį◊…ę≥ŃĶŪ…ķ≥…£¨ľī¬»ĽĮ“ÝĶńőÔ÷ ĶńŃŅ «14.35g°¬143.5g/mol£Ĺ0.1mol£¨’‚ňĶ√ų¬»ņŽ◊””–2łŲ «ŇšŐŚ£¨ŃŪÕ‚ĽĻ”–4łŲįĪ∆Ý∑÷◊” «ŇšŐŚ°£”…”ŕńŕĹÁő™’żįň√śŐŚĻĻ–ÕŇšņŽ◊”£¨ňý“‘É»ĹÁŅ…ń‹ĶńĹŠĻĻ”–ŃĹ÷÷£Ľ∆š÷–÷––ńņŽ◊”Co3£ęĶńľŘĶÁ◊”ŇŇ≤ľ Ĺő™3d6°£



| ńÍľ∂ | łŖ÷–Ņő≥Ő | ńÍľ∂ | ≥ű÷–Ņő≥Ő |
| łŖ“Ľ | łŖ“Ľ√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű“Ľ | ≥ű“Ľ√‚∑—Ņő≥ŐÕ∆ľŲ£° |
| łŖ∂Ģ | łŖ∂Ģ√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű∂Ģ | ≥ű∂Ģ√‚∑—Ņő≥ŐÕ∆ľŲ£° |
| łŖ»ż | łŖ»ż√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű»ż | ≥ű»ż√‚∑—Ņő≥ŐÕ∆ľŲ£° |
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņ“ņ«ķŐśű• «“Ľ÷÷∆§∑Ű≤°”√“©£¨ňŁŅ…“‘”…‘≠ŃŌXĺ≠Ļż∂ŗ≤Ĺ∑ī”¶ļŌ≥…°£
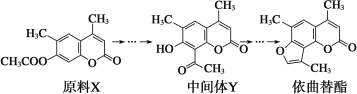
Ō¬Ń–ňĶ∑®≤Ľ’ż»∑Ķń «( )
A£ģ‘≠ŃŌX”Ž÷–ľšŐŚYĽ•ő™Õ¨∑÷“žĻĻŐŚ
B£ģ‘≠ŃŌXŅ…“‘ ĻňŠ–‘KMnO4»‹“ļÕ …ę
C£ģ÷–ľšŐŚYń‹∑Ę…ķľ”≥…°Ę»°īķ°ĘŌŻ»•°Ę—űĽĮ°ĘĽĻ‘≠∑ī”¶
D£ģ1 mol“ņ«ķŐśű•ń‹”Ž2 mol NaOH∑Ę…ķ∑ī”¶
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅTiO2ļÕTiCl4∂ľ «Ó—Ķń÷ō“™ĽĮļŌőÔ£¨ń≥ĽĮ—ß–ň»§–°◊ť‘ŕ Ķ—ť “∂‘ŃĹ’ŖĶń÷∆Īłľį–‘÷ ĹÝ––ŐĹĺŅ°£
ĘŮ£ģ÷∆ĪłTiCl4
Ķ—ť “ņŻ”√∑ī”¶TiO2 (s)£ęCCl4(g)![]() TiCl4(g)£ęCO2(g)£¨‘ŕőřňģőř—űŐűľĢŌ¬÷∆ĪłTiCl4£¨◊į÷√ÕľļÕ”–Ļō–ŇŌĘ»ÁŌ¬£ļ
TiCl4(g)£ęCO2(g)£¨‘ŕőřňģőř—űŐűľĢŌ¬÷∆ĪłTiCl4£¨◊į÷√ÕľļÕ”–Ļō–ŇŌĘ»ÁŌ¬£ļ
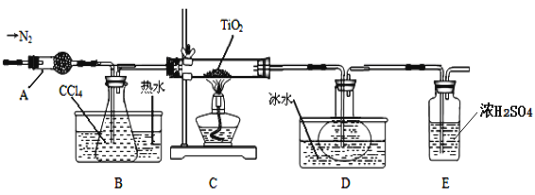
őÔ÷ | »ŘĶ„/°ś | ∑–Ķ„/°ś | ∆šňŻ |
CCl4 | °™23 | 76 | ”ŽTiCl4Ľ•»‹ |
TiCl4 | °™25 | 136 | ”Ų≥Ī ™Ņ’∆Ý≤ķ…ķį◊őŪ |
«ŽĽōīūŌ¬Ń–ő Ő‚£ļ
£®1£©“«∆ųAĶń√Ż≥∆ « °£
£®2£© Ķ—ťŅ™ ľ«įļůĶń≤Ŕ◊ųįŁņ®£ļĘŔľž≤ť◊į÷√∆Ý√‹–‘£¨Ęŕ◊ť◊į“«∆ų£¨ĘŘÕ®N2“Ľ∂ő Īľš£¨Ę‹ľ”◊į“©∆∑£¨Ę›Ķ„»ľ
ĺ∆ĺęĶ∆ĘřÕ£÷ĻÕ®N2ĘŖÕ£÷Ļľ”»»°£’ż»∑Ķń≤Ŕ◊ųň≥–Ú «__________________________°£ Ķ—ťĹŠ Ýļů”Ż∑÷ņŽD÷–Ķń“ļŐ¨ĽžļŌőÔ£¨ňý≤…”√≤Ŕ◊ųĶń√Ż≥∆ « °£
£®3£©◊į÷√Eń‹∑Ů £®ŐÓ°įń‹°ĪĽÚ°į≤Ľń‹°Ī£©ĽĽ≥…◊į÷√A£¨ņŪ”… « °£
£®4£©TiCl4 «÷∆»°ļĹŐžļĹŅ’Ļ§“Ķ≤ńŃŌ°™°™Ó—ļŌĹūĶń÷ō“™‘≠ŃŌ°£ń≥Ó—ļŌĹūĶń‘™ňōĽĻ”–AlļÕSiĶ»£¨“—÷™‘ŕ≥£ő¬Ō¬£¨Ó— «“Ľ÷÷ńÕ«ŅňŠ«ŅľÓĶńĹū Ű£¨«Ž…Ťľ∆ Ķ—ťľž—ť∆š÷–ĶńSi‘™ňō°£ °£
ĘÚ£ģ÷∆ĪłTiO2ľį≤‚∂®TiO2Ķń÷ ŃŅ∑÷ ż£ļ
‘ŕTiCl4÷–ľ”ňģ°Ęľ”»»£¨ňģĹ‚Ķ√ĶĹ≥ŃĶŪTiO2°§xH2O£¨ĺ≠Ļż¬ň°ĘňģŌī£¨‘Ŕļśł…°ĘĪļ…’≥ż»•ňģ∑÷Ķ√ĶĹ∑ŘŐŚTiO2°£
£®5£©–ī≥Ų…ķ≥…TiO2°§xH2OĶńĽĮ—ß∑Ĺ≥Ő Ĺ °£
£®6£©ľž—ť≥ŃĶŪ «∑ŮŌīĶ”ł…ĺĽĶń∑Ĺ∑® «___________________________________________°£
£®7£© “Ľ∂®ŐűľĢŌ¬£¨ĹęTiO2»‹Ĺ‚≤ĘĽĻ‘≠ő™Ti3+£¨”√NH4Fe(SO4)2ĪÍ◊ľ»‹“ļĶő∂®Ti3+÷Ń»ę≤Ņ…ķ≥…Ti4+°£Ķő∂®∑÷őŲ Ī£¨≥∆»°…Ō ŲTiO2 ‘—ý0.2g£¨ŌŻļń0.1 mol°§L£≠1 NH4Fe(SO4)2ĪÍ◊ľ»‹“ļ20mL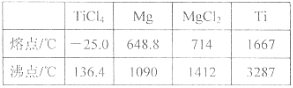 °£
°£
ĘŔŇš÷∆NH4Fe(SO4)2ĪÍ◊ľ»‹“ļ Ī£¨ľ”»Ž“Ľ∂®ŃŅH2SO4ĶńńŅĶń «£ļ °£
Ęŕł√Ķő∂®≤Ŕ◊ųňý”√ĶĹĶń÷ł ĺľŃ « °£
ĘŘ‘ÚTiO2÷ ŃŅ∑÷ żő™ °£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņ£®1£©CaC2÷–C22-”ŽO22+Ľ•ő™Ķ»ĶÁ◊”ŐŚ£¨O22+ĶńĶÁ◊” ĹŅ…ĪŪ ĺő™ £Ľ1 mol O22+÷–ļ¨”–Ķń¶–ľŁ żńŅő™____________________°£
£®2£©Fe2(SO4)3ĺßŐŚ÷–ŐķņŽ◊”ĶńļňÕ‚ĶÁ◊”ŇŇ≤ľ Ĺő™ °£
£®3£©““īľ”Ž““»©ĶńŌŗ∂‘∑÷◊”÷ ŃŅŌŗ≤Ó≤Ľīů£¨Ķę““īľĶń∑–Ķ„£®78.5°ś£©»īĪ»““»©Ķń∑–Ķ„£®20.8°ś£©łŖ≥Ų–Ū∂ŗ£¨∆š‘≠“Ú « °£
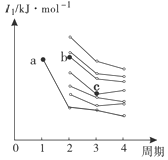
£®4£©”“Õľ «≤Ņ∑÷÷ų◊Ś‘™ňōĶŕ“ĽĶÁņŽń‹Ő›∂»Õľ£¨Õľ÷–aĶ„∂‘”¶Ķń‘™ňōő™«‚£¨b°ĘcŃĹĶ„∂‘”¶Ķń‘™ňō∑÷Īūő™ °Ę £®ŐÓ‘™ňō∑ŻļŇ£©°£
£®5£©—«ŌűňŠĶńňŠ–‘«Ņ”ŕīő¬»ňŠĶń‘≠“Úő™ °£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅŌ¬ĪŪ «‘™ňō÷‹∆ŕĪŪĶń“Ľ≤Ņ∑÷°£ĪŪ÷–ňýŃ–Ķń◊÷ńł∑÷ĪūīķĪŪń≥“Ľ÷÷ĽĮ—ß‘™ňō°£
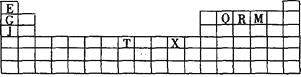

£®1£©–ī≥ŲĽýŐ¨T3+ĶńļňÕ‚ĶÁ◊”ŇŇ≤ľ Ĺ£ļ £ĽT‘ŕ÷‹∆ŕĪŪ÷–ňý‘ŕ∑÷«Ýő™ °£
£®2£©Q°ĘR°ĘMĶńĶŕ“ĽĶÁņŽń‹”…īůĶĹ–°Ķńň≥–Ú « (”√‘™ňō∑ŻļŇĪŪ ĺ)£¨‘≠“Ú °£
£®3£©Ō¬Ń–”–Ļō…Ō Ų‘™ňōĶńňĶ∑®’ż»∑Ķń « °£
A£ģJĪ»XĽÓ∆√£¨ňý“‘JŅ…“‘‘໋“ļ÷–÷√ĽĽ≥ŲX |
B£ģĹęJ2M2»‹”ŕňģ£¨“™∆∆ĽĶņŽ◊”ľŁļÕĻ≤ľŘľŁ |
C£ģRE3∑–Ķ„łŖ”ŕQE4£¨÷ų“™ «“Úő™«į’ŖŌŗ∂‘∑÷◊”÷ ŃŅĹŌīů |
D£ģ“ĽłŲQ2E4∑÷◊”÷–ļ¨”–őŚłŲ¶ńľŁļÕ“ĽłŲ¶–ľŁ |
£®4£©G2OĶń»ŘĶ„Ī»J2OĶń £®ŐÓ°įłŖ°ĪĽÚ°įĶÕ°Ī£© £¨∆š‘≠“Ú « °£
£®5£© G”ŽRĶ•÷ ÷ĪĹ”ĽĮļŌ…ķ≥…“Ľ÷÷ņŽ◊”ĽĮļŌőÔG3R°£ł√ĺßŐŚĺŖ”–ņŗň∆ ĮńęĶń≤„◊īĹŠĻĻ°£√Ņ≤„÷–£¨G‘≠◊”ĻĻ≥…∆Ĺ√śŃýĪŖ–ő£¨√ŅłŲŃýĪŖ–őĶń÷––ń”–“ĽłŲR‘≠◊”°£≤„”Ž≤„÷ģľšĽĻľ–‘”“Ľ∂® żŃŅĶń‘≠◊”°£«Žő ’‚–©ľ–‘”Ķń‘≠◊””¶ł√ « (ŐÓGĽÚRĶń‘™ňō∑ŻļŇ)°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņ°ĺĽĮ—ß°™°™—°–ř3£ļőÔ÷ ĹŠĻĻ”Ž–‘÷ °Ņ
A°ĘB°ĘC°ĘDő™«įňń÷‹∆ŕ‘™ňō°£A‘™ňōĶń‘≠◊”ľŘĶÁ◊”ŇŇ≤ľő™ns2np2£¨B‘™ňō‘≠◊”Ķń◊ÓÕ‚≤„ĶÁ◊” ż «∆šĶÁ◊”≤„ żĶń3Ī∂£¨C‘™ňō‘≠◊”ĶńMń‹≤„Ķńpń‹ľ∂”–3łŲőī≥…∂‘ĶÁ◊”£¨D‘™ňō‘≠◊”ļňÕ‚ĶńMń‹≤„÷–÷Ľ”–2∂‘≥…∂‘ĶÁ◊”°£«ŽĽōīūŌ¬Ń–ő Ő‚£ļ
£®1£©ĶĪn=2 Ī£¨AB2 Ű”ŕ__________∑÷◊”£®ŐÓ°įľę–‘°ĪĽÚ°į∑«ľę–‘°Ī£©£¨∑÷◊”÷–”–__________łŲ¶ńľŁ°Ę__________łŲ¶–ľŁ°£A6H6∑÷◊”÷–A‘≠◊”Ķń‘”ĽĮĻžĶņņŗ–Õ «__________‘”ĽĮ°£
£®2£©ĶĪn=3 Ī£¨A”ŽB–ő≥…ĶńĺßŐŚ Ű”ŕ__________ĺßŐŚ°£
£®3£©»ŰA‘™ňōĶń‘≠◊”ľŘĶÁ◊”ŇŇ≤ľő™3s23p2£¨A°ĘC°ĘD»ż÷÷‘™ňōĶńĶŕ“ĽĶÁņŽń‹”…īůĶĹ–°Ķńň≥–Ú «__________£®”√‘™ňō∑ŻļŇĪŪ ĺ£©£ģ
£®4£©“—÷™ń≥ļž◊Ō…ęŇšļŌőÔĶń◊ť≥…ő™CoCl3°§5NH3°§H2O£¨ł√ŇšļŌőÔ÷–Ķń÷––ńņŽ◊”Ó‹ņŽ◊”‘ŕĽýŐ¨ ĪĶńļňÕ‚ĶÁ◊”ŇŇ≤ľ Ĺő™____________________°£
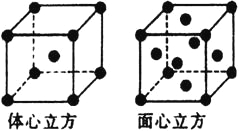
£®5£©Ĺū ŰŐķĶńĺßŐŚ‘ŕ≤ĽÕ¨ő¬∂»Ō¬”–ŃĹ÷÷∂—Ľż∑Ĺ Ĺ£¨ĺßįŻ∑÷Īū»ÁÕľňý ĺ£¨ŐŚ–ńŃĘ∑ĹĺßįŻļÕ√ś–ńŃĘ∑ĹĺßįŻ÷– Ķľ ļ¨”–ĶńFe‘≠◊”łŲ ż÷ģĪ»ő™__________°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅĹę ŃŅAgBrĻŐŐŚ»‹‘ŕňģ÷–£¨»‹“ļ÷–īś‘ŕAgBr(s)![]() Ag£ę(aq)£ęBr£≠(aq)°£‘ÚŌ¬Ń–ňĶ∑®’ż»∑Ķń «£® £©
Ag£ę(aq)£ęBr£≠(aq)°£‘ÚŌ¬Ń–ňĶ∑®’ż»∑Ķń «£® £©
A£ģŌÚīňŐŚŌĶ÷–Ķőľ”◊„ŃŅŇ®NaCl»‹“ļ£¨∑ĘŌ÷≥ŃĶŪ◊™ĽĮő™į◊…ę£¨ňĶ√ųKsp(AgCl)£ľKsp(AgBr)
B£ģń≥»‹“ļ÷–c(Ag£ę)°§c(Br£≠)=Ksp(AgBr)£¨ňĶ√ųīň Īc(Ag£ę)=c(Br£≠)
C£ģŌÚīňŐŚŌĶ÷–ľ”»Ž…ŔŃŅAgBrĻŐŐŚ£¨∆Ĺļ‚’żŌÚ“∆∂Į£¨ĶĪ‘Ŕīő∆Ĺļ‚ Īc(Ag£ę)°Ęc(Br£≠)‘Ųīů
D£ģīňŐŚŌĶ÷–“Ľ∂®īś‘ŕc(Ag£ę)=c(Br£≠)=[Ksp(AgBr)]1/2
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņń≥Õ¨—ßŌŽÕ®ĻżĪ»ĹŌŃĹ÷÷◊ÓłŖľŘ—űĽĮőÔĶńňģĽĮőÔĶńňŠ–‘«Ņ»űņī—ť÷§ŃÚ”ŽŐľĶń∑«Ĺū Ű–‘«Ņ»ű£¨ňŁ≤…”√Ńň»ÁÕľňý ĺĶń◊į÷√ĹÝ–– ‘—ť°£«ŽĽōīū£ļ
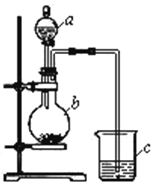
£®1£©“«∆ųaĶń√Ż≥∆ «____________£¨”¶ Ę∑ŇŌ¬Ń–“©∆∑÷–Ķń_______°£
A£ģŌ°ŃÚňŠ B£ģ—«ŃÚňŠ C£ģ«‚ŃÚňŠ D£ģ—őňŠ
£®2£©“«∆ųbĶń√Ż≥∆ «____________£¨”¶ Ę∑ŇŌ¬Ń–“©∆∑÷–Ķń______°£
A£ģŐľňŠł∆ B£ģŃÚňŠń∆ C£ģ¬»ĽĮń∆ D£ģŐľňŠń∆
£®3£©“«∆ųc÷–”¶ Ę∑ŇĶń“©∆∑ «____________£¨»ÁĻŻŅīĶĹĶńŌ÷Ōů «____________________£¨÷§√ųb÷–∑ī”¶≤ķ…ķŃň_____________________£¨ľīŅ…÷§√ų__________Ī»_________ňŠ–‘«Ņ£¨∑«Ĺū Ű–‘__________Ī»__________«Ņ£¨b÷–∑Ę…ķ∑ī”¶ĶńņŽ◊”∑Ĺ≥Ő Ĺő™__________________________________________
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅĻ§“Ķ…ŌŅ…”√ ≥—őļÕ ĮĽ“ Įő™÷ų“™‘≠ŃŌ£¨ĺ≠≤ĽÕ¨Ķń∑Ĺ∑®…ķ≥…īŅľÓ°£«ŽĽōīūŌ¬Ń–ő Ő‚£ļ
£®1£©¬¨≤ľņľ∑® «“‘ ≥—ő°Ę ĮĽ“ Į°ĘŇ®ŃÚňŠ°ĘĹĻŐŅő™‘≠ŃŌ£¨‘ŕłŖő¬Ō¬ĹÝ––ž—…’£¨‘ŔĹĢ»°°ĘĹŠĺß∂Ý÷∆Ķ√īŅľÓ°£
ĘŔ ≥—őļÕŇ®ŃÚňŠ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™£ļ __________________________________£Ľ
ĘŕŃÚňŠń∆ļÕĹĻŐŅ°Ę ĮĽ“ Į∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™£ļ__________________________ (“—÷™ŃÚňŠń∆◊Ų—űĽĮľŃ£¨…ķ≥…őÔ÷–∆ÝŐŚ÷Ľ”–“Ľ÷÷)°£
£®2£©įĪľÓ∑®ĶńĻ§“’»Á”“Õľňý ĺ£¨Ķ√ĶĹĶńŐľňŠ«‚ń∆ĺ≠ž—…’…ķ≥…īŅľÓ°£
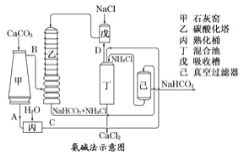
ĘŔÕľ÷–Ķń÷–ľš≤ķőÔC «___________£¨(ŐÓĽĮ—ß Ĺ£¨Ō¬Õ¨)D «___________£Ľ
Ęŕ◊į÷√““÷–∑Ę…ķ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™___________________£Ľ
£®3£©Ń™ļŌ÷∆ľÓ∑® «∂‘įĪľÓ∑®ĶńłńĹÝ£¨∆š”ŇĶ„ «≥żŃňłĪ≤ķőÔ¬»ĽĮÔßŅ…”√◊ųĽĮ∑ Õ‚ĽĻ”–_______________°£
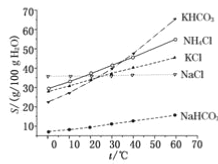
£®4£©”–»ň»Ōő™ŐľňŠ«‚ľō”ŽŐľňŠ«‚ń∆ĶńĽĮ—ß–‘÷ Ōŗň∆£¨Ļ “≤Ņ…”√įĪľÓ∑®“‘¬»ĽĮľōļÕ ĮĽ“ ĮĶ»ő™‘≠ŃŌ÷∆ŐľňŠľō°£«ŽĹŠļŌŌ¬ÕľĶń»‹Ĺ‚∂»(S)ňśő¬∂»ĪšĽĮ«ķŌŖ£¨∑÷őŲňĶ√ų «∑ŮŅ…––£Ņ_______________°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ļķľ —ß–£”Ň—° - Ń∑Ōį≤ŠŃ–ĪŪ - ‘Ő‚Ń–ĪŪ
ļĢĪĪ °Ľ•Ń™ÕÝő•∑®ļÕ≤ĽŃľ–ŇŌĘĺŔĪ®∆ĹŐ® | ÕÝ…Ō”–ļ¶–ŇŌĘĺŔĪ®◊®«Ý | ĶÁ–Ň’©∆≠ĺŔĪ®◊®«Ý | …śņķ ∑–ťőř÷ų“Ś”–ļ¶–ŇŌĘĺŔĪ®◊®«Ý | …ś∆ů«÷»®ĺŔĪ®◊®«Ý
ő•∑®ļÕ≤ĽŃľ–ŇŌĘĺŔĪ®ĶÁĽį£ļ027-86699610 ĺŔĪ®” Ōš£ļ58377363@163.com