
����Ҫ��ش����и���
�������� ���� ��NH3������������Һ ��Һ̬�Ȼ��� ��AgCl����ޱ����� �����ǣ���ջش�����ţ���
���������У�1�����ڵ���ʵ��� ����2�����ڷǵ���ʵ��� ��
��3������ǿ����ʵ��� ����4���ܵ������ ��
����д�����з�Ӧ�Ļ�ѧ����ʽ��
����NaHCO3���ɵĻ��Ϸ�Ӧ
�� ��MgCl2�μӵķֽⷴӦ
�� ��Fe2O3�μӵ��û���Ӧ
�� ��HNO3���ɵĸ��ֽⷴӦ
(��).ͬ��ͬѹ�����£�ͬ�����CH4��SO2������֮���� ��ͬ������CH4��SO2�����֮���� ������������ԭ�Ӹ���������ȣ���CH4��SO2������֮���� ��
����������Ƭ��ʯī��������a��b���ַ�ʽ����ʢ��ϡ��������Һ�ͷ�̪��Һ�����Һ�IJ��������У�����һ��ʱ������ȹ۲쵽��Һ���������� ������ţ���
| A����͢� | B����͢����� | C����͢� | D����͢����� |
����12�֣�ÿ��1�֣�����1���ܢݢ��� 2���ڢ���3���ܢ���4���٢�����2NaOH+CO2+H2O=2NaHCO3��NaOH+CO2=NaHCO3 �� MgCl2 Mg+Cl2���� Fe2O3+2Al
Mg+Cl2���� Fe2O3+2Al 2Fe+Al2O3 �� AgNO3+HCl=HNO3+AgCl���������֣�(��).1��4 4��1 3��20 �� ������B
2Fe+Al2O3 �� AgNO3+HCl=HNO3+AgCl���������֣�(��).1��4 4��1 3��20 �� ������B
�������������������ʺͷǵ���ʶ��ǻ���������ǰ���½��з��������Ϊ��1���ܢݢޣ� 2���ڢ� ��3���ܢ� ��4���٢� ��������ϵƽʱ��ѧ�Ļ���֪ʶ����ɻ�ѧ����ʽ�����ʾ��(��).ͬ��ͬѹ�����£�ͬ�����CH4��SO2�����ʵ�����ͬ����������֮����Ħ������֮�ȣ���Ϊ1��4 ��ͬ������CH4��SO2�����֮������Ħ�������ķ��ȡ�����������ԭ�Ӹ���������ȣ����趼Ϊ1molת��ΪCH4��SO2������֮����3��20 ��������a�ǵ��أ�bΪԭ��أ���a���൱�ڵ��ˮ���������������ӷŵ磬��Χ�ʼ��ԣ�������������ʴ����̼����Χ��������������������ȹ۲쵽��Һ���������Ǣ�͢�������
���㣺���⿼���˸��������»�ѧ��Ӧ����ʽ����д�͵绯ѧ��Ӧԭ����


 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2����NO3-��SO42-��Cl����I����HCO3-��ȡ����Һ��������ʵ�飺
| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���Ӽ��μ�����Һ | ��Һ���ɫ |
| ��ȡ��������Һ������ͭƬ��Ũ���ᣬ���� | ����ɫ������������������Ա�ɺ���ɫ |
| ��ȡ��������Һ������BaCl2��Һ | �а�ɫ�������� |
| ��ȡ���е��ϲ���Һ������AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���� |
| ��ȡ��������Һ������NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ�����������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)���ڵ��۵⻯����Һ�У��μ������������Ƽ�����Һ�������ῴ����Һ����ɫ��������Ϊ________�����ӷ���ʽ__________________________��
���ڵ�͵����γɵ���ɫ��Һ�У��μ��������Ƽ�����Һ��������ɫ����ʧ��������Ϊ________________________�����ӷ���ʽ��_______________________��
�۶ԱȢٺ͢�ʵ�����õĽ������I2��ClO����SO42������������ǿ������˳������Ϊ________________________________________��
(2)������Ƭ��ͭƬ�����ʵ��֤��������ʵ����д����Ӧ�Ļ�ѧ����ʽ��
��Ũ����������Ա�ϡ����ǿ��___________________________��
���Ȼ�����Һ��Fe3���������Ա�����ͭ��Һ�е�Cu2��ǿ��__________________________________________��
�����Ļ�ԭ�Ա�ͭǿ��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D�������ʾ�Ϊ����������ɵĿ����Ի����������������ʵ����ӣ����Ӳ����ظ���ϣ��У�
| ������ | Na+��Al3+��Ba2+��NH4+ |
| ������ | Cl����OH����CO32����SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ũ�ȷֱ�Ϊ1 mol/L��FeCl3��FeCl2��CuCl2�����Һ100 mL������һ���������ۣ������������ա�
��1����ַ�Ӧ�������Һ�л���һ������Cu2��������Һ��һ�����еĽ������ӻ���___________������������Һ�е����ʵ�����ΧΪ________________�����ܺ��еĽ������������Ϊ____________��
��2����Ӧ��Ϻ�������ʣ�࣬��Һ��һ�����еĽ�������Ϊ___________��Ϊ______mol��
һ��û�еĽ�������Ϊ______________��
��3������FeCl3��Һ�м�����������ᣬ������Ӧ�����ӷ���ʽΪ____________________��
��4��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ�������ʣ�S��H2S��HNO3��NO��H2O���÷�Ӧ�Ļ�ѧ����ʽΪ________________________________������Ӧ������ת����0.3mol���ӣ������������������___________g�����ɵ������ڱ���µ������________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������������ǿ�������������ɷ�����H1N1���С�
��1����̼������һ���ж�����;��������ϵ��̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ�
��H2O2��ʱ����Ϊ��ҵ��Һ���������������ɿ�ҵ��Һ�е��軯��(��NaCN)�������·�Ӧʵ�֣�NaCN��H2O2��H2O=A��NH3������������A�Ļ�ѧʽ______________
��ijǿ���Է�Ӧ��ϵ�У���Ӧ��������ﹲ�������ʣ�
O2��MnO4-��H2O��Mn2����H2O2��H������֪�÷�Ӧ��H2O2ֻ���������¹��̣�H2O2�� O2��
д���÷�Ӧ�����ӷ���ʽ��_______________________________________________��
��2��ij��Ȼ��Ļ�ѧʽ�ɱ�ʾΪ:aNa2CO3��bNaHCO3��2H2O��ȡm g��Ȼ������ˮ�����Һ��������Һ����μ���1 mol/L�����ᣬ��״���²�����CO2������������������֮��Ĺ�ϵijͬѧ��������ͼ��ʾ��A��B���ߣ��Իش��������⣺
��_______������ȷ����Ȼ��Ļ�ѧʽΪ___________��
�ڼ���������CO2�������(��״��)�����ֵΪ _____________mL��
��3�� ����������ȱλ����пZnFe2Oy��������NOx��Ⱦ��ʹNOxת��ΪN2��ͬʱZnFe2Oyת��ΪZnFe2O4����2 mol ZnFe2Oy������NO2������0.5 mol N2����y��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С���û�����(FeS2)�������ƺ�������Һ��Ϸ�Ӧ�Ʊ�ClO2���壬����ˮ���ո�����ɵ�ClO2��Һ���ڴ˹�������Ҫ�������˵��¶ȣ����¶Ȳ���������Ӧ���ӣ�Ӱ������ClO2����Ĵ��ȣ��һ�Ӱ��ClO2�������ʣ����������ͼ��ʾ��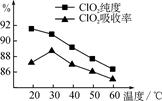
��1�� ��ͼ��֪����Ӧʱ��Ҫ���Ƶ������¶���________�棬Ҫ�ﵽ��Ҫ����Ҫ��ȡ�Ĵ�ʩ��______________��
��2�� ��֪���������е���Ԫ�������������¿ɱ�ClO3-������SO42-����д��FeS2�������ƺ�������Һ��Ϸ�Ӧ���ɶ�������(ClO2)�����ӷ���ʽ��______________________��
��3�� ��С�����ԡ�m(ClO2)/m(NaClO3)����Ϊ����ClO2���ʵ�ָ�ꡣ��ȡNaClO3��Ʒ6.0 g��ͨ����Ӧ�����ջ��400 mL ClO2��Һ��ȡ����Һ20 mL��37.00 mL 0.500 mol��L��1 (NH4)2Fe(SO4)2��Һ��ַ�Ӧ������Fe2������0.050 0 mol��L��1 K2Cr2O7����Һ�ζ����յ㣬����K2Cr2O7����Һ20.00 mL����Ӧԭ��Ϊ��
4H����ClO2��5Fe2��=Cl����5Fe3����2H2O
14H����Cr2O72-��6Fe2��=2Cr3����6Fe3����7H2O
�Լ���ClO2�ġ����ʡ�(д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Т���Ƭ ��NaCl��Һ �۰�ˮ �ܴ��� �ݾƾ� ������ ��H2SO4
��KOH���� ������ ��KAl(SO4)2��12H2O�������ܵ������ �����ڵ���ʵ���____ ___�����ڷǵ���ʵ��� ����������� �����ڼ���� ��������ţ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣��������ʣ� ������ ��Br2 ��Na2O ��Ba(OH)2 ��CO2 ��SO3
��NaCl��Һ ��NaCl ��HCl ��H2SO4
���ڵ���ʵ��� �� ���ڷǵ���ʵ��� ���ܵ������ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com