

| ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol��L-1HCl��Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 0.00 | 26.29 |
| 2 | 25.00 | 1.56 | 31.30 |
| 3 | 25.00 | 1.00 | 27.31 |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��c��K+��+c��H+��=c(HC2O4-)+c(OH-)+c(C2O42-) |
| B��c(HC2O4-)+ c (C2O42-)+ c��H2C2O4��=0��1mol/L |
| C��c(H+)��c��OH-�� |
| D��c��K+��=c��H2C2O4��+c(HC2O4-)+c(C2O42-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1/2H2��g��+1/2Cl2��g��=HCl��g������H =��92��3kJ/mol |
| B��CH4��g��+2O2��g��=CO2��g��+2H2O��g������H =��802��3kJ/mol |
| C��2H2��g��+O2��g��=2H2O��l������H =��571��6kJ/mol |
| D��CO��g��+1/2O2��g��=CO2��g������H =��283kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ˮ�ܡ����ܡ�̫���ܡ������ܡ����ܵ�����Դ������ʹ��ú��ʯ�͵Ȼ�ʯȼ�� |
| B��������չũ���������������Ľո�ת��Ϊ����Ч����Դ |
| C����������ú��ʯ�ͺ���Ȼ���������㾭�÷�չ����Ҫ |
| D��������չ����Դ���������϶����������綯�����ȣ��Լ���̼������������ŷ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c��OHһ��>c��H+��ʱ��a=b | B��c��K+��>c��CH3COO����ʱ��a>b |
| C��c��OHһ��=c��H+��ʱ��a>b | D��c��K+��<c��CH3COO����ʱ��a<b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
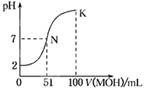
| A��HAΪ���ᣬMOHΪǿ�� |
| B��pH=7ʱ��HA��MOHǡ����ȫ��Ӧ |
| C����N�㣬c(A-)=c(M+) + c(MOH) |
| D����K�㣬c(M+)>c(A-)>c(OH-)>c(H+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٣��ڣ��ۣ��� | B���ڣ��٣��ܣ��� | C���ۣ��ܣ��ڣ��� | D���ܣ��ۣ��ڣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c(H+)=c(CH3COO��)+c(OH��) |
| B����ˮϡ�͵�ԭ�����10������ҺpH��Ϊ4 |
| C���������������ƹ��壬��ҺpH���� |
| D��������pH=11��NaOH��Һ��Ϻ�������Һ��c(Na+)=c(CH3COO��) |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com