

| B2H6 |
| H2O2/OH |
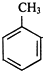 ��Cl2��Ӧ����EΪ
��Cl2��Ӧ����EΪ ��
�� ����ˮ������FΪ
����ˮ������FΪ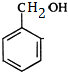 ��D��F����������Ӧ����GΪ
��D��F����������Ӧ����GΪ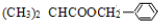 ���ݴ˴��⣻
���ݴ˴��⣻ ��Cl2��Ӧ����EΪ
��Cl2��Ӧ����EΪ ��
�� ����ˮ������FΪ
����ˮ������FΪ ��D��F����������Ӧ����GΪ
��D��F����������Ӧ����GΪ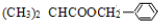 ��
��| Cu/Ag |
| �� |

| Ũ���� |
| �� |
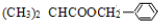 +H2O��
+H2O��| Cu/Ag |
| �� |

| Ũ���� |
| �� |
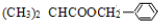 +H2O��
+H2O��

 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.1s | B��2.5s |
| C��5s | D��10s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ͭ��ϡ���ᷴӦ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O | ||||
| B����������ͭ��Һ��Ӧ��Cu2++2Na=2Na++Cu | ||||
| C����NaHCO3��Һ�м��������Ba��OH��2��Һ��HCO3-+Ba2++OH-=BaCO3��+H2O | ||||
D���ö��Ե缫��ⱥ���Ȼ�����Һ��2Cl-+2H2O
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ����ϡ������п��Ӧ�ⶨ��Ӧ���ʵ�װ�ã��ڷ�Һ©���м���ϡ���ᣬ����ƿ�м���п��ͨ���ⶨ����һ�����������õ�ʱ�����ⶨ��Ӧ�����ʣ�����50mL 1mol/L���ᣬ����ƿ�м������и���п����������ͬ��������H2�����ǣ�������
��ͼ��ʾ����ϡ������п��Ӧ�ⶨ��Ӧ���ʵ�װ�ã��ڷ�Һ©���м���ϡ���ᣬ����ƿ�м���п��ͨ���ⶨ����һ�����������õ�ʱ�����ⶨ��Ӧ�����ʣ�����50mL 1mol/L���ᣬ����ƿ�м������и���п����������ͬ��������H2�����ǣ�������| A������п�� |
| B������� |
| C��������ͭ���ʵ�п�� |
| D��������ͭ���ʵ�п�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij����С��������ͼ��ʾ��װ����ȡCl2���ṩ���Լ���Ũ���ᡢ����ʳ��ˮ��Cl2�������ܽ�Ƚ�С��������������Һ��������ع��壬��Ӧ�Ļ�ѧ����ʽΪ��2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O
ij����С��������ͼ��ʾ��װ����ȡCl2���ṩ���Լ���Ũ���ᡢ����ʳ��ˮ��Cl2�������ܽ�Ƚ�С��������������Һ��������ع��壬��Ӧ�Ļ�ѧ����ʽΪ��2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| n(CO2) |
| n(H2O) |
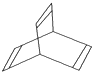
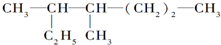 ��������
��������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com