
����Ŀ����ˮ������������ѧʵ�����г����Ļ�ѧ�Լ��������ڹ�ũҵ������Ҳ���й㷺Ӧ�á�ij�о���ѧϰС��Ϊ�ⶨ��ˮ��Ũ�ȣ����ð�ˮ��Ϊ�ᴿ����ʱ���Լ��������������������ʵ�����£�
�������ϣ�
�ټ��ȵı�ɫ��Χ��pH��3.1��ɫ��pH��3.1��4.4��ɫ�� pH��4.4��ɫ
�ڷ�̪�ı�ɫ��Χ��pH��8.2��ɫ ��pH��8.2��10.0�ۺ�ɫ��pH��10.0��ɫ
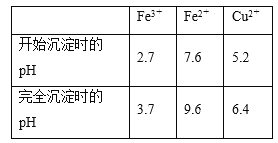
����֪��Fe3����Fe2����Cu2��ת��Ϊ��������ʱ��Ӧ��pH���±���
ʵ��һ���궨��ˮ��Ũ��
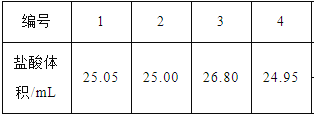
ȡ25.00mLԼΪ0.10 mol��L��1��ˮ����ƿ�У���0.1000 mol��L��1������еζ���ʵ�������������±���ʾ��
��1���ζ�����ˮ������ӷ���ʽΪ____________________________________���ɴ˿���֪ѡ��ĵζ�ָʾ��ӦΪ__________________��������������������̪����
��2���ð�ˮ��ȷŨ��Ϊ____________________mol��L��1������ȷ��С�������λ��
��3�����3����Һ������Ũ���ɴ�С��˳��Ϊ__________________________________��
ʵ��� �ᴿ��������
ijѧϰС��ͬѧ��Ӻ�FeSO4��Fe2(SO4)3���ʵ�CuSO4��Һ���ᴿ����������Ҫʵ�鲽�����£�
��һ�� �����Һ�м���3% H2O2��Һ��ַ�Ӧ���ټ���ϡ��ˮ������ҺpH�����ˡ�
�ڶ��� ����Һ�м���ϡ���������ҺpH��1��2���ᴿ������
��4������3% H2O2��Һ��������___________________________��
��5����ϡ��ˮ����pHӦ������Χ___________________________��
��6���������ʿ��������ϡ��ˮ����___________________________��������ţ�
A��NaOH B��Cu(OH)2 C��CuO D��NaHCO3
���𰸡�NH4����H2O![]() NH3��H2O��H�� ���� 0.1000 [Cl��]>[NH4��]>[H��]>[OH��] ��[Cl��] [NH4��] [H��] [OH��] ����Һ�е�Fe2������ΪFe3�������Һ�е�FeSO4����ΪFe2��SO4��3 5.2>pH��3.7 ��3.7��pH<5.2 ��[3.7��5.2�� BC
NH3��H2O��H�� ���� 0.1000 [Cl��]>[NH4��]>[H��]>[OH��] ��[Cl��] [NH4��] [H��] [OH��] ����Һ�е�Fe2������ΪFe3�������Һ�е�FeSO4����ΪFe2��SO4��3 5.2>pH��3.7 ��3.7��pH<5.2 ��[3.7��5.2�� BC
��������
(1).������ζ���ˮ���ζ������Ȼ����ǿ�������Σ���Һ�����ԣ�Ҫѡ�������Է�Χ�ڱ�ɫ��ָʾ����
��2������c(NH3��H2O)V(NH3��H2O)=c(HCl)V(HCl)���㰱ˮ��Ũ�ȣ�
��3�����3��ʵ�������������Һ�к����Ȼ�李����
��4����ȥ��Һ�е���Ԫ�أ���������������ͭ��������Ҫ��Fe2������ΪFe3����
��5������Fe3����ȫ������Cu2�����ܳ������жϵ���pH�ķ�Χ��
��6�����������ϡ��ˮ�����ʣ���Ҫ���ϵ������ǣ���ʹ��ҺPH���߶������������ʡ�
(1). �ζ������Ȼ����ǿ�������Σ�笠�����ˮ�⣬��Һ�����ԣ�ˮ������ӷ���ʽ��NH4����H2O![]() NH3��H2O��H�����Ȼ����Һ�����ԣ�Ҫѡ�������Է�Χ�ڱ�ɫ��ָʾ������ѡ���ȣ�
NH3��H2O��H�����Ȼ����Һ�����ԣ�Ҫѡ�������Է�Χ�ڱ�ɫ��ָʾ������ѡ���ȣ�
��2��������ʵ������ƫ����������Χ��ȥ������1��2��4�������ݣ�ƽ����������������25.00mL��c(NH3��H2O)V(NH3��H2O)=c(HCl)V(HCl)��c(NH3��H2O)= 0.1000 mol��L��1��0.025L��0.025L=0.1000 mol��L��1��
��3�����3��ʵ�������������Һ�к����Ȼ�李����ᣬ��������Ũ��[Cl��]>[NH4��]>[H��]>[OH��]��
��4����ȥ��Һ�е���Ԫ�أ���������������ͭ��������Ҫ��Fe2������ΪFe3�������������Һ�м���3% H2O2��Һ�������ǰ���Һ�е�Fe2������ΪFe3����
��5��ʹFe3����ȫ������Cu2�����ܳ��������Ե���pH�ķ�Χ��3.7��pH<5.2��
��6��Cu(OH)2��CuO��ʹ��ҺPH���߶������������ʣ����������ϡ��ˮ��NaOH��NaHCO3���������ʣ����ܴ���ϡ��ˮ����ѡBC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ������������⣺
��1����ʽ2 -��ϩ�Ľṹ��ʽ____________________��
��2����H2�ӳ�����2��5 - ���������Ȳ����ϵͳ����____________________��
��3��![]() ��ϵͳ����____________________��
��ϵͳ����____________________��
��4��ij���ķ�����Ϊ72����������Ӧ���ɵ�һ�ȴ���ֻ��һ�֣������Ľṹ��ʽΪ_______��
��5��д���ɱ�ϩ�Ʊ��۱�ϩ�Ļ�ѧ��Ӧ����ʽ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬ�¡�ͬѹ�£���A�����г���O2��B�����г���O3��
��1������������������ȣ���A������B�������ݻ�֮����_______________________��
��2����������������ԭ��������ȣ���A������B�������ݻ�����___________________________��
��3�����������������Ϊ3��2����O2��O3���ʵ���֮��Ϊ________________������֮��Ϊ_____________���ܶ�֮��Ϊ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ������������ԭ��Ӧ���ǣ� ��
A.3 NO2 + H2O = 2 HNO3 + NOB.FeCl3��3NaOH��3NaCl��Fe(OH)3��
C.2KMnO4![]() K2MnO4 + MnO2 + O2��D.Cl2 + 2NaOH �TNaClO + NaCl + H2O
K2MnO4 + MnO2 + O2��D.Cl2 + 2NaOH �TNaClO + NaCl + H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����Ҫ��NaCl��������100mL 2.0mol��L-1��NaCl��Һ���Իش����и��⣺
(1)���������У������õ����� _____________��
A����ƿ B��100mL����ƿ C����Ͳ D����ͷ�ι� E��50mL����ƿ F����ƽ
(2)��Ҫʵʩ���ƣ������������⣬��ȱ����������Ʒ��___________________��
(3)����ƿ��ʹ��ǰ����____________________________________��
(4)���dz������ƹ��̼���Ϊ���¸����裺
A����ȴ B������ C��ϴ�� D������ E���ܽ� F��ҡ�� G��ת��
����ȷ�IJ���˳��Ӧ��_____________________________(����������)��
(5)������Ϻ�ʦָ������λͬѧ������������ijһ��������������Ϊ�������������ᵼ��������ҺŨ��ƫ�ߵ��� ��_________��
A������ NaCl����ʱ���������
B������ʱ��������ƿ�̶���
C�����ܽ���ȴ����Һת������ƿ���ֱ��ת�붨�ݲ���
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ�
(6)ͨ������ɵó�����������ƽ��ȡNaCl����������______________������5.0mol/L��NaCl��Һ����Ӧ����Ͳ��ȡ________________mL����Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵������ȷ���ǣ� ��
A.��״���£�22.4 L���������к��еķ�����ΪNA
B.0.5 mol��L-1��Na2SO4��Һ�У�����Na���ĸ���ΪNA
C.��״���£�22.4L����������22.4L����������ԭ��������Ϊ2NA
D.32 g ������������ԭ����ĿΪ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��3H2O2 + 2H2CrO4 =2 Cr(OH)3 + 3O2 ��+ 2H2O
��1������Cr��___________��H2O2��____________����
��2��_____________���������_______________������ԭ��Ӧ��
��3����Ӧת����0.3mol���ӣ�������������ڱ�״̬�����ԼΪ__________��
��4���õ����ű�������ת�Ƶķ������Ŀ��_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������m gij���壬����Ħ������ΪM g��mol-1���������ӵ�������NA��ʾ����
��1������������ʵ���Ϊ________mol��
��2���������ڱ���µ��ܶ�Ϊ__________g/L��
��3���������ڱ�״���µ����Ϊ____________L��
��4������������0.1Lˮ�У�ˮ���ܶȣ�1g��mL-1,�Ҳ����Ƿ�Ӧ��������Һ�����ʵ���������Ϊ_____��
��5������������ˮ���γ�VL��Һ������Һ�����ʵ���Ũ��Ϊ_____mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Һ�У���������ˮ�������ɵ�H��Ũ��֮�ȣ��١âڡâۡâܣ���
�� 1 mol��L��1�����ᡡ �� 0.1 mol��L��1������
�� 0.01 mol��L��1��NaOH��Һ�� �� 0.001 mol��L��1��NaOH��Һ
A. 1��10��100��1 000B. 0��1��12��11C. 14��13��12��11D. 14��13��2��3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com