
| 2.2 |
| 44 |
| 1.12 |
| 28 |
| 2.2 |
| 44 |
| 1.12 |
| 28 |
| 8.74 |
| 437 |


 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д� ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
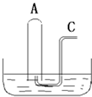 ��ͼ��ʾ����B����װ��500mLˮ���ݻ�Ϊa mL���Թ�A������NO2��NO�Ļ�����壨��״���������Թ�A������B�۵�ˮ�У���ַ�Ӧ���Թ�A��������������Ϊ0.5amL��
��ͼ��ʾ����B����װ��500mLˮ���ݻ�Ϊa mL���Թ�A������NO2��NO�Ļ�����壨��״���������Թ�A������B�۵�ˮ�У���ַ�Ӧ���Թ�A��������������Ϊ0.5amL���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������������ȼ�����Ȼ��⣬�Ȼ������ȷֽ�Ϊ���������� |
| B�������������ڸ��¡���ѹ�����������¿������ɰ�����ͬʱ�����ַֽ�Ϊ���������� |
| C������������û����⣬�����ֿ����û����� |
| D��������ˮ��Ӧ��������ʹ����ᣬ��������������¿ɷֽ�Ϊ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ش��� | K | L | M | Q | R | T | N |
| ԭ�Ӱ뾶/nm | 0.186 | 0.160 | 0.143 | 0.102 | 0.089 | 0.074 | 0.152 |
| ��Ҫ���ϼ� | +1 | +2 | +3 | +6��-2 | +2 | -2 | +1 |
| A����RCl2�У���ԭ������������8���ӵ��ȶ��ṹ |
| B��Ԫ��L��N����������Ϊ����ͬһ���� |
| C��K��M��Q��Ԫ������������Ӧ��ˮ��������֮��ɷ�����ѧ��Ӧ |
| D���⻯��е㣺H2Q��H2T |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| CaO |
| �� |
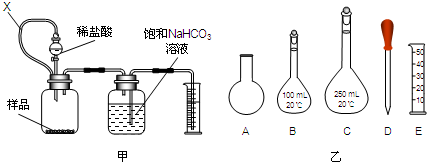
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com