
����Ŀ������N��P��Fe��Ti��Ԫ�ص����Ͳ������Ź㷺����;��
(1)��̬Feԭ��δ�ɶԵ�����Ϊ______������̬Tiԭ�ӵļ۵����Ų�ͼ��_____________��
(2)�����������ѧ�ģ�FuNvio Cacace���˻���˼��������о������N4���ӣ����е�ԭ�ӵĹ���ӻ���ʽΪ__________________________��
(3)�Ƚ���̬�⻯���(PH3)�Ͱ�(NH3)�ļ��ǣ�PH3________NH3(����ڡ�����С�ڡ����ڡ�)����Ҫԭ��Ϊ____________________________________________________________��
(4)����Ľṹ����M�ܴ���ϩ����ϩ������ϩ�ľۺϣ���ṹ��ͼ��ʾ��

�����M��Ԫ���У��縺��������___________(������)��
��M���_________(����)��
A ���� B ����
C ���Ӽ� D ��λ��

(5)��֪���ʯ�ľ���������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼ����ͼB��ʾ�������������������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼӦ����ͼ__________(����)��

(6)ij�ִ��Ե������ľ����ṹ��ͼ��ʾ�����о�����ԭ���������ԭ�ӵĸ���Ϊ____________�������������ױ߳�Ϊa cm����Ϊc cm�������ִ��Ե������ľ����ܶ�Ϊ__________g��cm-3(�ú�a��c��NA�ļ���ʽ��ʾ)��
���𰸡�4 ![]() sp3 С�� �縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ�� �� ABD A 12
sp3 С�� �縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ�� �� ABD A 12 ![]()
��������
(1) FeΪ26��Ԫ�أ���̬Fe�ĺ�������Ų�ʽΪ1s22s22p63s23p63d64s2����̬ԭ��δ�ɶԵ�����Ϊ4����TiΪ22��Ԫ�أ���̬Ti�ĺ�������Ų�ʽΪ1s22s22p63s23p63d24s2�����Ի�̬Tiԭ�ӵļ۵����Ų�ͼ��![]() ���𰸣�4��
���𰸣�4��![]() ��
��
��2��N4���ӵĿռ乹����P4���ƣ�4��Nԭ���γ��������幹�ͣ�ÿ��Nԭ���γ�3��N-N����������1�Թµ��Ӷԣ��ӻ������ĿΪ4����Nԭ�Ӳ�ȡsp3�ӻ����𰸣�sp3��
(3) ��Ϊ�縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ������PH3С��NH3���𰸣�С�ڣ��縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ��
(4) �����M��Ԫ����Ti��C��H��O��Cl ������O�ķǽ�������ǿ���ǽ�����Խǿ�縺��Խ�����Ե縺�������������𰸣�����
��M����̼̼˫����̼̼������C��H����C��O���ȣ�����Ϊ![]() ���� ˫���к�1��
���� ˫���к�1��![]() ����1������������M�Ľṹ֪Ti��O֮������λ����û�����Ӽ�����ѡABD��
����1������������M�Ľṹ֪Ti��O֮������λ����û�����Ӽ�����ѡABD��
(5) )�ɽ��ʯ�ľ����ṹ��֪�����ʯ�ľ����൱��һ��������������ѻ�����һ��С�����������ѻ����������ľ���Ϊ���������ѻ������ݽ��ʯ�ľ���������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼ֪���������ľ���������Խ��ߴ�ֱ��ֽ���ϵ�ͶӰͼΪA���𰸣�A��
(6) ���ݾ�̯�����ڵ����������У�����Nԭ����Ϊ2��Feԭ����Ϊ2��1/2+12��1/6+3=6�����Ե������Ļ�ѧʽFe3N��������ԭ���������ԭ�ӵĸ���Ϊ12���������ױ߳�Ϊacm����Ϊccm�����������![]() a2ccm3���������ִ��Ե������ľ����ܶ�Ϊ
a2ccm3���������ִ��Ե������ľ����ܶ�Ϊ![]() g��
g��![]() a2ccm3=
a2ccm3=![]() g/cm3���𰸣�12��
g/cm3���𰸣�12��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ���ѧ�ҷ�����һ�����ɹ̵������-�����ε�أ����ɴ���Li+������������ʣ���ص��ܷ�ӦΪ![]()
![]()
![]() ������˵����ȷ����
������˵����ȷ����

A. �̵�ʱ��﮵缫������ԭ��Ӧ
B. �ѵ�ʱ���ɸ��ϵ缫�ĵ缫��Ӧ��2Li3N-6e-=6Li++N2��
C. �̵�ʱ�����·�е������ɸ��ϵ缫����﮵缫
D. �ѵ�ʱ��Li+���ɸ��ϵ缫Ǩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ����������Ϊ��̬��ƽ����ϵ��ƽ�ⳣ������ʽΪK��![]() ���йظ�ƽ����ϵ��˵���������
���йظ�ƽ����ϵ��˵���������
A. �����¶ȣ��÷�Ӧƽ�ⳣ��K�ı仯���ж�
B. ����ѹǿ��W������������С
C. �÷�Ӧ�Ļ�ѧ����ʽΪ3Z��g����2W��g��![]() X��g����2Y��g��
X��g����2Y��g��
D. ����X�����Ũ��ƽ��������Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CH4��H2��C�������ʵ���Դ���ʣ�����ȼ�յ��Ȼ�ѧ����ʽΪ��
��CH4(g)��2O2(g)=CO2(g)��2H2O(l) ��H����890.3kJ��mol��1
��2H2(g)��O2(g)=2H2O(l) ��H����571.6kJ��mol��1
��C(s)��O2(g)=CO2(g) ��H����393.5kJ��mol��1
��1������д���һ�ּ���ϸ������������øʹ������O2���ò���������������ϸ��ʹ1mol��������CO2������Һ̬ˮ���ų�������________(����������������������)890.3kJ��
��2��������CO2�����ںϳ�ˮú��(��Ҫ�ɷ���һ����̼������)��CH4��CO2=2CO��2H2��1gCH4��ȫ��Ӧ���ͷ�15.46kJ����������
���ܱ�ʾ�÷�Ӧ�����������仯����________(����ĸ)��
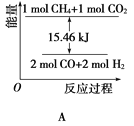



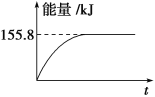
���������ʵ�����Ϊ1mol��CH4��CO2����ij�����ܱ������У���ϵ�ų�����������ʱ��ı仯��ͼ��ʾ����CH4��ת����Ϊ________��
��3��C(s)��H2 (g)����Ӧ������C(s)��2H2(g)=CH4(g)�ķ�Ӧ����ֱ�Ӳ�������ͨ��������Ӧ�����C(s)��2H2(g)=CH4(g)�ķ�Ӧ����H��_____��
��4��Ŀǰ���������������ʵ��о���ȼ���о����ص㣬���й��������������ʵ��о������п��е���_______(����ĸ)��
A��Ѱ�����ʴ�����ʹCO2��H2O��Ӧ����CH4��O2�����ų�����
B��Ѱ�����ʴ������ڳ��³�ѹ��ʹCO2�ֽ�����̼��O2
C��Ѱ�����ʴ���������̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳ�ˮú��(CO��H2)
D������̬̼�ϳ�ΪC60����C60��Ϊȼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С���ͬѧ����ʵ������п��Ũ���ᷴӦ��ʵ��ʱ����ͬѧ��Ϊ�����������Ƕ���������ͬѧ��Ϊ���������������⣬�����ܲ���������Ϊ����֤�ס�����λͬѧ���ж��Ƿ���ȷ����ͬѧ�������ͼ��ʾʵ��װ��(п��Ũ���Ṳ��ʱ����������ΪX���Ҹ�װ����ȥ)���Իش��������⣺

��1��������Ӧ�����ɶ�������Ļ�ѧ����ʽΪ__��
��2����ͬѧ��Ϊ�����ܲ���������������__��
��3����ͬѧ�ڰ�װ��װ�ú������Ƚ��е�һ��������__��
��4��A�м�����Լ�������__��������__��B�м�����Լ�������__��������__��E�м�����Լ�������__��������__��
��5������֤������X�к���������ʵ�������ǣ�
C��__��
D��__��
���ȥ��װ��B�����ܷ����D�е������ж�����X����������__(������������������)��ԭ����_��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2S��CO2�ڸ����·�����Ӧ��H2S(g)��CO2(g) ![]() COS(g)��H2O(g)����610 Kʱ����0.10 mol CO2��0.40 mol H2S����2.5 L�Ŀո�ƿ�У���Ӧƽ���ˮ�����ʵ�������Ϊ0.02��
COS(g)��H2O(g)����610 Kʱ����0.10 mol CO2��0.40 mol H2S����2.5 L�Ŀո�ƿ�У���Ӧƽ���ˮ�����ʵ�������Ϊ0.02��
����Ӧ�����ٷֱ�����������壬��ʹH2Sת�����������________(����)��
A. H2S B. CO2 C.COS D.N2
����620 K�ظ�ʵ�飬ƽ���ˮ�����ʵ�������Ϊ0.03���÷�Ӧ�Ħ�H________0(�>����<������)��
��H2S��ƽ��ת���ʦ�1��_____%����Ӧƽ�ⳣ��K��______�����ڢ�С��Ҫд������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��CO(g) + H2O(g) ![]() H2(g) + CO2(g) ��ƽ�ⳣ��K���¶ȵı仯���±�������˵����ȷ������ ��
H2(g) + CO2(g) ��ƽ�ⳣ��K���¶ȵı仯���±�������˵����ȷ������ ��
�¶�/�� | 400 | 500 | 830 | 1000 |
ƽ�ⳣ��K | 10 | 9 | 1 | 0.6 |
A. �÷�Ӧ������Ӧ�����ȷ�Ӧ
B. ����ʱ����ѹǿ������Ӧ��������
C. 830��ʱ����Ӧ�ﵽƽ�⣬һ����c(CO)��c(CO2)
D. 400��ʱ������CO2���ʵ���Խ�࣬ƽ�ⳣ��KԽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ��������ԭ�Ϻϳɻ�״��E���{���ӻ�����H��ʾ��ͼ��ͼ��ʾ��
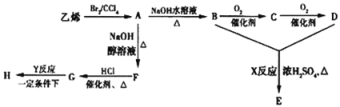
��ش��������⣺
��1��д���������ʵĽṹ��ʽ��B______��G_______
��2��д�����·�Ӧ�ķ�Ӧ���ͣ�X_____��Y______��
��3��д�����·�Ӧ�Ļ�ѧ����ʽ��A��B��_______
��4������״��E��NaOHˮ��Һ���ȣ�������Ӧ�Ļ�ѧ����ʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ���������������������Դ���õ�������أ�����˵����ȷ����
A. ���ס����ס�С���еĵ��۾�ˮ��ɱ���Ҵ�
B. ���ö�����̼����ȫ�������ϣ����Ի�������ЧӦ
C. �ⶨ�������Ƶ��۵�ʱ�����Խ��������ƹ������ʯӢ�����и��¼���
D. �����к��зḻ�Ŀ����Դ�������������������Ի��![]() ��
��![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com