
 ��g��+1/2O
��g��+1/2O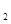 ��g����CO��g��+2H
��g����CO��g��+2H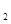 ��g��
��g��  H1����35.6kJ��mol
H1����35.6kJ��mol
 ��g��+2O
��g��+2O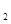 ��g����CO2��g��+2H2O��g��
��g����CO2��g��+2H2O��g��  H2����890.3kJ��mol
H2����890.3kJ��mol
 ��g��+CO
��g��+CO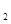 ��g����2CO��g��+2H
��g����2CO��g��+2H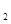 ��g��
��g��  H3��247.3kJ��mol
H3��247.3kJ��mol
| ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
| c(NH3)/( mol ��/L-1) | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |
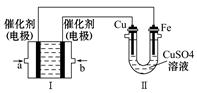
 �����ʵ���Ũ�� ��
�����ʵ���Ũ�� �� ��g��+H2O��g����CO��g��+3H2��g��
��g��+H2O��g����CO��g��+3H2��g�� H��250.3kJ��mol
H��250.3kJ��mol ��3�֣�����ÿ��2��
��3�֣�����ÿ��2��| | N2+3H2 2NH3 2NH3 | ||
| ʼ̬mol/L | 0.5 | 1.3 | 0 |
| ��Ӧmol/L | 0.1 | 0.3 | 0.20 |
| ��̬mol/L | 0.4 | 1.0 | 0.20 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NO(g) DH��a kJ��mol��1��ƽ�ⳣ��K���±���
2NO(g) DH��a kJ��mol��1��ƽ�ⳣ��K���±���| �¶�/�� | 1538 | 1760 | 2404 |
| ƽ�ⳣ��K | 0.86��10��4 | 2.6��10��4 | 64��10��4 |
 2NO(g)�ﵽƽ��ʱN2��Ũ��Ϊ �������¶��²�����O2��NO�ķ�Ӧ��������������λ��Ч���֣�
2NO(g)�ﵽƽ��ʱN2��Ũ��Ϊ �������¶��²�����O2��NO�ķ�Ӧ��������������λ��Ч���֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��4Q1+0.5Q2 | B��4Q1+Q2+10Q3 | C��4Q1+2Q2 | D��4Q1+0.5Q2+9Q3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��488.3 | B����244.15 | C��244.15 | D����488.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
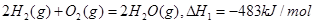 ��������Ȼ�ѧ����ʽ��
��������Ȼ�ѧ����ʽ��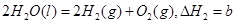 ������˵����ȷ���ǣ� ��
������˵����ȷ���ǣ� ��| A���Ȼ�ѧ��Ӧ����ʽ�л�ѧ��������ʾ���Ӹ��� |
B���÷�Ӧ��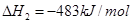 |
C��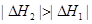 |
D��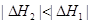 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2HBr(g) ��H=��72kJ/mol������1mol Br2(1)��Ҫ���յ�����Ϊ30kJ����������������±���
2HBr(g) ��H=��72kJ/mol������1mol Br2(1)��Ҫ���յ�����Ϊ30kJ����������������±���| | H2(g) | Br2(g) | HBr(g) |
| ����/kJ��mol��1 | 436 | a | 369 |
 l����ȫȼ�������ȶ�������ų�������ΪY kJ����1molP��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=________________________________��
l����ȫȼ�������ȶ�������ų�������ΪY kJ����1molP��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=________________________________���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com