
ЁОЬтФПЁПИљОнЬтвтЭъГЩЯТСаЮЪЬтЁЃ
ЃЈ1ЃЉНЋЕШжЪСПЕФаПЗлЗжБ№ЭЖШы10 mL 0.1 molЁЄLЃ1бЮЫсКЭ10 mL 0.1 molЁЄLЃ1 CH3COOHШмвКжаЁЃШєаПВЛзуСПЃЌЗДгІПьЕФЪЧ________ЃЈЬюаДбЮЫсШмвКЁЂДзЫсШмвКЃЉЁЃ
ЃЈ2ЃЉНЋЕШСПЕФаПЗлЗжБ№ЭЖШыcЃЈHЃЋЃЉОљЮЊ1 molЁЄLЃ1ЁЂЬхЛ§ОљЮЊ10 mLЕФбЮЫсКЭДзЫсШмвКжаЁЃШєаПВЛзуСПЃЌЗДгІПьЕФЪЧ________ЃЈЬюаДбЮЫсШмвКЁЂДзЫсШмвКЃЉЁЃ
ЃЈ3ЃЉЩшЫЎЕФЕчРыЦНКтЯпШчЭМЫљЪОЁЃ
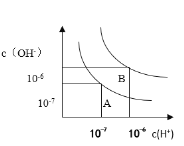
a.ШєвдAЕуБэЪО25ЁуЪБЫЎдкЕчРыЦНКтЪБЕФСЃзгХЈЖШЃЌЕБЮТЖШЩ§ИпЕН100ЁуЪБЃЌЫЎЕФЕчРыЦНКтзДЬЌЕНBЕуЃЌдђДЫЪБЫЎЕФРызгЛ§діМгЕН____________ЃЛ
b.НЋpH=8ЕФBaЃЈOHЃЉ2ШмвКгыpH=5ЕФЯЁбЮЫсЛьКЯЃЌВЂБЃГждк100ЁуЕФКуЮТЃЌгћЪЙЛьКЯШмвКЕФpH=7ЃЌдђBa(OH) 2ШмвККЭбЮЫсЕФЬхЛ§БШЮЊ___________ЁЃ
ЃЈ4ЃЉ25ЁцЪБЃЌCH3COOHгыCH3COONaЕФЛьКЯШмвКЃЌШєВтЕУЛьКЯШмвКpHЃН6ЃЌдђШмвКжаcЃЈCH3COOЃЃЉЃcЃЈNaЃЋЃЉЃН________ mol/L ЃЈЬюзМШЗЪ§жЕЃЉЁЃ
ЃЈ5ЃЉвбжЊNaHSO4дкЫЎжаЕФЕчРыЗНГЬЪНЮЊNaHSO4=NaЃЋЃЋHЃЋЃЋSO42ЃЁЃФГЮТЖШЯТЃЌЯђcЃЈHЃЋЃЉЃН1ЁС10Ѓ6 molЁЄLЃ1ЕФеєСѓЫЎжаМгШыNaHSO4ОЇЬхЃЌБЃГжЮТЖШВЛБфЃЌВтЕУШмвКЕФcЃЈHЃЋЃЉЃН1ЁС10Ѓ2 molЁЄLЃ1ЁЃЯТСаЖдИУШмвКЕФа№Ъіе§ШЗЕФЪЧ________________ЁЃ
AЃЎИУЮТЖШИпгк25 Ёц
BЃЎгЩЫЎЕчРыГіРДЕФHЃЋЕФХЈЖШЮЊ1ЁС10Ѓ10 molЁЄLЃ1
CЃЎШЁИУШмвКМгЫЎЯЁЪЭ100БЖЃЌШмвКжаЕФЫЎЕчРыГіЕФcЃЈHЃЋЃЉМѕаЁ
DЃЎМгШыNaHSO4ОЇЬхвжжЦЫЎЕФЕчРы
ЁОД№АИЁПбЮЫсШмвК ДзЫсШмвК 10-12 2:9 99ЁС10Ѓ8mol/L ABD
ЁОНтЮіЁП
ЃЈ1ЃЉИљОнЧтРызгХЈЖШДѓаЁХаЖЯЃЛ
ЃЈ2ЃЉИљОнЧтРызгХЈЖШДѓаЁХаЖЯЃЛ
ЃЈ3ЃЉИљОнc(HЃЋ)ЁЂc(OH-)БфЛЏХаЖЯKwЕФБфЛЏЃЛ
ЃЈ4ЃЉИљОнЕчКЩЪиКуc(HЃЋ)+c(Na+)= c(OH-)+c(CH3COO-)НјааМЦЫуЃЛ
ЃЈ5ЃЉИљОнЫЎЕФЕчРыЦНКтНјааЗжЮіЁЃ
ЃЈ1ЃЉСНжжвЛдЊЫсШмжЪЕФЮяжЪЕФСПЯрЕШЃЌЕЋбЮЫсжаc(HЃЋ)ДѓЃЌЙЪбЮЫсгыаПЗДгІЕФЫйТЪПьЃЛ
ЃЈ2ЃЉетСНжжЫсЕФc(HЃЋ)ЯрЕШЃЌЯдШЛКѓепЕФЮяжЪЕФСПХЈЖШДѓЃЌШмжЪЕФЮяжЪЕФСПвВДѓЃЌГѕЪМЕФЫйТЪЯрЭЌЃЌЕЋЫцзХЗДгІЕФНјааЃЌHЃЋВЛЖЯБЛЯћКФЃЌШѕЫсCH3COOHЕФЕчРыЦНКтВЛЖЯе§ЯђвЦЖЏЃЌгжЕчРыГіHЃЋЃЌЙЪдкЗДгІНјааЕФЭЌвЛЪБПЬCH3COOHШмвКжаЕФc(HЃЋ)ДѓгкбЮЫсШмвКжаЕФc(HЃЋ)ЃЌЫљвдДзЫсгыаПЗДгІЫйТЪПьЃЛ
ЃЈ3ЃЉa.25ЁцЪБДПЫЎжаc(HЃЋ)=c(OH-)=10-7 mol/LЃЌKw= c(HЃЋ)c(OH-)=10-14ЃЌЕБЮТЖШЩ§ИпЕН100ЁцЃЌДПЫЎжаc(HЃЋ)= c(OH-)=10-6 mol/LЃЌдђKw= c(HЃЋ)c(OH-)=10-12ЃЌДгAЕуЕНBЕуЃЌЫЎЕФРызгЛ§Дг10-14діМгЕН10-12ЃЛ
b. 100ЁцЪБЃЌНЋpH=8ЕФBa(OH)2ШмвКжаЃКc(OH-)=10-4 mol/LЃЌpH=5ЕФЯЁбЮЫсжаЃКc(HЃЋ)=10-5 mol/LЃЌЩшЧтбѕЛЏБЕЕФЬхЛ§ЮЊxЃЌбЮЫсЕФЬхЛ§ЮЊyЃЌ100ЁцЕФКуЮТЃЌЛьКЯШмвКpH=7ЃЌШмвКГЪМюадЃЌдђШмвКжаЧтбѕИљРызгХЈЖШЮЊЃК![]() =10-5 mol/LЃЌ
=10-5 mol/LЃЌ
дђЃКc(OH-)=![]() =10-5 mol/LЃЌНтЕУxЃКy=2ЃК9ЃЛ
=10-5 mol/LЃЌНтЕУxЃКy=2ЃК9ЃЛ
ЃЈ4ЃЉ25ЁцЪБЃЌCH3COOHгыCH3COONaЕФЛьКЯШмвКЃЌШєВтЕУЛьКЯШмвКpHЃН6ЃЌЛьКЯШмвКЯдЫсадЃЌдђc(HЃЋ)= 10-6 mol/LЃЌc(OH-)=10-8mol/LЃЌШмвКжаЕчКЩЪиКуЮЊc(HЃЋ)+c(Na+)= c(OH-)+c(CH3COO-)ЃЌдђc(CH3COO-)-c(Na+)= c(HЃЋ)- c(OH-)=10-6 mol/L -10-8mol/L=99ЁС10Ѓ8mol/LЃЛ
ЃЈ5ЃЉAЃЎ25ЁцЪБДПЫЎжаc(HЃЋ)=1ЁС10-7mol/LЃЌc(HЃЋ)=1ЁС10-6mol/LЫЕУїДйНјСЫЫЎЕФЕчРыЃЌЙЪT>25ЁцЃЌбЁЯюAе§ШЗЃЛ
BЃЎpH=6ЃЌЫЎЕФРызгЛ§ГЃЪ§ЮЊ1ЁС10-12ЃЌЫЎЕФРызгЛ§ГЃЪ§=ЧтРызггыЧтбѕИљХЈЖШЕФГЫЛ§ЃЌШмвКЕФpHЮЊ2ЃЌЙЪгЩЫЎЕчРыГіРДЕФc(HЃЋ)=1ЁС10-10mol/LЃЌбЁЯюBе§ШЗЃЛ
CЃЎЮТЖШВЛБфЪБЃЌKwВЛБфЃЌМгЫЎЯЁЪЭc(HЃЋ)МѕаЁЃЌKw= c(HЃЋ)ЁСc(OH-)ЃЌЫљвдc(OH-)діДѓЃЌбЁЯюCДэЮѓЃЛ
DЃЎNaHSO4ЕФЕчРыЩњГЩЧтРызгЃЌЖдЫЎЕФЕчРыЦ№вжжЦзїгУЃЌЫЎЕФЕчРыГЬЖШМѕаЁЃЌбЁЯюDе§ШЗЃЛ
Д№АИбЁABDЁЃ


 аЁбЇбЇЯАКУАяЪжЯЕСаД№АИ
аЁбЇбЇЯАКУАяЪжЯЕСаД№АИ аЁбЇЭЌВНШ§СЗКЫаФУмОэЯЕСаД№АИ
аЁбЇЭЌВНШ§СЗКЫаФУмОэЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЮЊВтЖЈФГЬўAЕФЗжзгзщГЩКЭНсЙЙ,ЖдетжжЬўНјаавдЯТЪЕбщ:
ЂйШЁвЛЖЈСПЕФИУЬў,ЪЙЦфГфЗжШМЩеКѓЕФЦјЬхЭЈЙ§зАгаCaCl2ИЩдяЙм,ИЩдяЙмдіжи7.2g;дйЭЈЙ§ЪЏЛвЫЎ,ЪЏЛвЫЎдіжи17.6gЁЃ
ЂкОВтЖЈ,ИУЬў(ЦјЬх)дкБъзМзДПіЯТЕФУмЖШЮЊ1.25gЁЄL-1ЁЃ
ЯжвдAЮЊжївЊдСЯКЯГЩввЫсввѕЅ,ЦфКЯГЩТЗЯпШчЭМ1ЫљЪОЁЃ
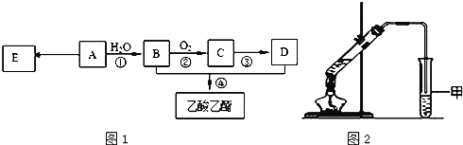
(1)0.1molИУЬўAФмгы _____gфхЗЂЩњМгГЩЗДгІ;МгГЩВњЮяаш _____molфхеєЦјЭъШЋШЁДњ;
(2)BжаЙйФмЭХЕФУћГЦЪЧ_____, BПЩЭЈЙ§МгШыЧПбѕЛЏМСЮЊ_______(ШЮЬювЛжж)вЛВНжБНгбѕЛЏЮЊDЁЃ
(3)EЪЧГЃМћЕФИпЗжзгВФСЯ,аДГіEЕФНсЙЙМђЪН______;КЯГЩEЕФЗДгІРраЭ______;
(4)ФГЭЌбЇгУШчЭМ2ЫљЪОЕФЪЕбщзАжУжЦШЁЩйСПввЫсввѕЅЁЃЪЕбщНсЪјКѓ,ЪдЙмМзжаЩЯВуЮЊЭИУїЕФЁЂВЛШмгкЫЎЕФгЭзДвКЬхЁЃ
ЂйЪЕбщПЊЪМЪБ,ЪдЙмМзжаЕФЕМЙмВЛЩьШывКУцЯТЕФдвђЪЧ__________;
ЂкЩЯЪіЪЕбщжаБЅКЭЬМЫсФЦШмвКЕФзїгУЪЧ________;
ЂлдкЪЕбщЪвРћгУBКЭDжЦБИввЫсввѕЅЕФЪЕбщжа,ШєгУ1molBКЭ1molDГфЗжЗДгІ,ВЛФмЩњГЩ1molввЫсввѕЅ,двђЪЧ_____ЁЃ
(5)БШввЫсввѕЅЯрЖдЗжзгжЪСПДѓ14ЕФѕЅга_____жжНсЙЙЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТУцЪЧМИжжгаЛњЛЏКЯЮяЕФзЊЛЛЙиЯЕЃК
ЧыЛиД№ЯТСаЮЪЬтЃК
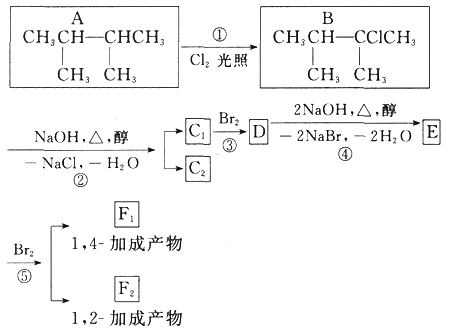
ЃЈ1ЃЉИљОнЯЕЭГУќУћЗЈЃЌЛЏКЯЮяAЕФУћГЦЪЧ_____ЁЃ
ЃЈ2ЃЉЩЯЭМЗДгІжаЃЈЬюЗДгІРраЭЃЉЃЌЂйЪЧ_____ЗДгІЃЌЂлЪЧ_____ЗДгІЁЃ
ЃЈ3ЃЉEЪЧживЊЕФЛЏЙЄдСЯЃЌаДГігЩDЩњГЩEЕФЛЏбЇЗНГЬЪНЃК_____ЁЃ
ЃЈ4ЃЉC1ЕФНсЙЙМђЪНЪЧ_____ЁЃ
ЃЈ5ЃЉаДГіЗћКЯЯТСаЬѕМўЕФAЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪН_____ЁЃ
ЂйКЌжЇСД ЂкКЫДХЙВеёЧтЦзга3ИіЗх
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈЛђБэЪОЗНЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A.ЕШЮяжЪЕФСПЕФСђеєЦјКЭСђЙЬЬхЗжБ№ЭъШЋШМЩеЃЌКѓепЗХГіШШСПЖр
B.гЩH+ЃЈaqЃЉ+OH-ЃЈaqЃЉ=H2OЃЈlЃЉЁїH=-57.3kJЁЄmol-1ПЩжЊЃЌШєНЋКЌ1 mol CH3COOHЕФЯЁШмвКгыКЌ1 mol NaOHЕФЯЁШмвКЛьКЯЃЌЗХГіЕФШШСПаЁгк57.3 kJ
C.гЩCЃЈЪЏФЋЃЉ==CЃЈН№ИеЪЏЃЉЁїH=+1.90 kJЁЄmol-1ПЩжЊЃЌН№ИеЪЏБШЪЏФЋЮШЖЈ
D.500ЁцЁЂ30MPaЯТЃЌНЋ0.5molN2ЃЈgЃЉКЭ1.5mol H2ЃЈgЃЉжУгкУмБеШнЦїжаГфЗжЗДгІЩњГЩNH3ЃЈgЃЉЃЌЗХШШ19.3kJЃЌЦфШШЛЏбЇЗНГЬЪНЮЊЃКN2ЃЈgЃЉ+3H2ЃЈgЃЉ![]() 2NH3ЃЈgЃЉЁїH=-38.6kJЁЄmol-1
2NH3ЃЈgЃЉЁїH=-38.6kJЁЄmol-1
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЂё.вбжЊвЛбѕЛЏЬМгыЫЎеєЦјЗДгІЙ§ГЬЕФФмСПБфЛЏШчЭМЫљЪОЃК
ЃЈ1ЃЉЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ___________________________ЁЃ
ЃЈ2ЃЉвбжЊЃКTiЃЈsЃЉ +2Cl2ЃЈgЃЉ = TiCl4ЃЈlЃЉ ЁїH = Ѓ804.2kJ/molЃЛ
2NaЃЈsЃЉ +Cl2ЃЈgЃЉ = 2NaClЃЈsЃЉ ЁїH = Ѓ882.0kJ/mol
NaЃЈsЃЉ = NaЃЈlЃЉ ЁїH =+2.6 kJ/mol
ЧыаДГігУвКЬЌФЦгыЫФТШЛЏюбжУЛЛГіюбЕФШШЛЏбЇЗНГЬЪН_____________________________________
II.дкФГвЛШнЛ§ЮЊ2 LЕФУмБеШнЦїФкЃЌМгШы0.8 molЕФH2КЭ0.6 molЕФI2ЃЌдквЛЖЈЬѕМўЯТЗЂЩњШчЯТЗДгІЃКH2ЃЈgЃЉЃЋI2ЃЈgЃЉ![]() 2HIЃЈgЃЉЁЁІЄH<0ЃЌЗДгІжаИїЮяжЪЕФХЈЖШЫцЪБМфБфЛЏЧщПіШчЯТЭММзЫљЪОЃК
2HIЃЈgЃЉЁЁІЄH<0ЃЌЗДгІжаИїЮяжЪЕФХЈЖШЫцЪБМфБфЛЏЧщПіШчЯТЭММзЫљЪОЃК

ЃЈ3ЃЉИУЗДгІЕФЛЏбЇЦНКтГЃЪ§БэДяЪНЮЊ________ЁЃ
ЃЈ4ЃЉИљОнЭММзЪ§ОнЃЌЗДгІПЊЪМжСДяЕНЦНКтЪБЃЌЦНОљЫйТЪvЃЈHIЃЉЮЊ________ЁЃ
ЃЈ5ЃЉЗДгІДяЕНЦНКтКѓЃЌЕк8ЗжжгЪБЃК
ЂйШєЩ§ИпЮТЖШЃЌЛЏбЇЦНКтГЃЪ§K________ЃЈЬюЁАдіДѓЁБЁАМѕаЁЁБЛђЁАВЛБфЁБЃЉЃЌHIХЈЖШЕФБфЛЏе§ШЗЕФЪЧ________ЃЈгУЭМввжаaЁЋcЕФБрКХЛиД№ЃЉЁЃ
ЂкШєМгШыI2ЃЌH2ХЈЖШЕФБфЛЏе§ШЗЕФЪЧ________ЃЈгУЭМввжаdЁЋfЕФБрКХЛиД№ЃЉЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЩшNAЮЊАЂЗќйЄЕТТоГЃЪ§ЕФжЕЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A.УмБеШнЦїжа2molSO2КЭ1molO2ДпЛЏЗДгІКѓЗжзгЪ§ЮЊ2NA
B.БъзМзДПіЯТЃЌ5.6LCO2ЦјЬхжаКЌгаЕФбѕдзгЪ§ФПЮЊ0.5NA
C.1molжиЫЎгы1molЫЎжажазгЪ§БШЮЊ2ЃК1
D.БъзМзДПіЯТЃЌ2.24LN2КЭO2ЕФЛьКЯЦјЬхжаЗжзгЪ§ЮЊ0.2NA
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊВтЖЈжаКЭШШЕФЪЕбщВНжшШчЯТЃК
![]() СПШЁ50mL
СПШЁ50mL![]() СђЫсЕЙШыаЁЩеБжаЃЌВтСПЮТЖШЃЛ
СђЫсЕЙШыаЁЩеБжаЃЌВтСПЮТЖШЃЛ
![]() СПШЁ50mL
СПШЁ50mL![]() NaOHШмвКЃЌВтСПЮТЖШЃЛ
NaOHШмвКЃЌВтСПЮТЖШЃЛ
![]() НЋNaOHШмвКЕЙШыаЁЩеБжаЃЌЛьКЯОљдШКѓВтСПЛьКЯвКЮТЖШЃЎ
НЋNaOHШмвКЕЙШыаЁЩеБжаЃЌЛьКЯОљдШКѓВтСПЛьКЯвКЮТЖШЃЎ
ЧыЛиД№ЃК
ЃЈ1ЃЉ![]() ШмвКЩдЙ§СПЕФдвђ______ЃЎ
ШмвКЩдЙ§СПЕФдвђ______ЃЎ
ЃЈ2ЃЉМгШыNaOHШмвКЕФе§ШЗВйзїЪЧ______![]() ЬюзжФИ
ЬюзжФИ![]() ЃЎ
ЃЎ
A.биВЃСЇАєЛКТ§МгШы![]() вЛДЮбИЫйМгШы
вЛДЮбИЫйМгШы![]() ЗжШ§ДЮМгШы
ЗжШ§ДЮМгШы
ЃЈ3ЃЉЪЙСђЫсгыNaOHШмвКЛьКЯОљдШЕФе§ШЗВйзїЪЧ______ЃЎ
ЮТЖШ ЪЕбщДЮЪ§ | Ц№ЪМЮТЖШ | жежЙЮТЖШ
| ЮТЖШВюЦНОљжЕ
| ||
| NaOH | ЦНОљжЕ | |||
1 |
|
|
|
|
|
2 |
|
|
|
|
|
3 |
|
|
|
|
|
ЃЈ4ЃЉЩшШмвКЕФУмЖШОљЮЊ![]() ЃЌжаКЭКѓШмвКЕФБШШШШн
ЃЌжаКЭКѓШмвКЕФБШШШШн![]() ЃЌЧыИљОнЪЕбщЪ§ОнЧѓГіжаКЭШШЮЊ______аДГіИУЗДгІЕФШШЛЏбЇЗНГЬЪН______
ЃЌЧыИљОнЪЕбщЪ§ОнЧѓГіжаКЭШШЮЊ______аДГіИУЗДгІЕФШШЛЏбЇЗНГЬЪН______
ЃЈ5ЃЉШєНЋКЌ![]()
![]() ЕФХЈСђЫсгыКЌ1molNaOHЕФШмвКЛьКЯЃЌЗХГіЕФШШСП______
ЕФХЈСђЫсгыКЌ1molNaOHЕФШмвКЛьКЯЃЌЗХГіЕФШШСП______![]() ЬюЁАаЁгкЁБЁЂЁАЕШгкЁБЛђЁАДѓгкЁБ
ЬюЁАаЁгкЁБЁЂЁАЕШгкЁБЛђЁАДѓгкЁБ![]() ЃЌдвђЪЧ______ЃЎ
ЃЌдвђЪЧ______ЃЎ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙЄвЕЩњВњСђЫсНгДЅЪвЗЂЩњШчЯТЛЏбЇЗДгІЃК2SO2(g)ЃЋO2(g)![]() 2SO3(g) ІЄHЃНЃ196.6kJЁЄmolЃ1ЁЃвЛЖЈЮТЖШЯТЃЌЯђМзЁЂввЁЂБћШ§ИіШнЛ§ОљЮЊ2LЕФКуШнУмБеШнЦїжаЭЖШыSO2(g)КЭO2(g)ЃЌЦфЦ№ЪМЮяжЪЕФСПМАSO2ЕФЦНКтзЊЛЏТЪШчБэЫљЪОЃЌЯТСаХаЖЯжаЃЌе§ШЗЕФЪЧЃЈ ЃЉ
2SO3(g) ІЄHЃНЃ196.6kJЁЄmolЃ1ЁЃвЛЖЈЮТЖШЯТЃЌЯђМзЁЂввЁЂБћШ§ИіШнЛ§ОљЮЊ2LЕФКуШнУмБеШнЦїжаЭЖШыSO2(g)КЭO2(g)ЃЌЦфЦ№ЪМЮяжЪЕФСПМАSO2ЕФЦНКтзЊЛЏТЪШчБэЫљЪОЃЌЯТСаХаЖЯжаЃЌе§ШЗЕФЪЧЃЈ ЃЉ
Мз | вв | Бћ | ||
Ц№ЪМЮяжЪЕФСП | n(SO2)/mol | 0.6 | 1.2 | 1.2 |
n(O2)/mol | 0.36 | 0.36 | 0.72 | |
SO2ЕФЦНКтзЊЛЏТЪ | 80% | ІС1 | ІС2 | |
A.МзЁњввЃЌЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌІС(O2)діДѓЃЌЗХГіЕФШШСПЮЊ47.18kJ
B.ЦНКтЪБЃЌБћжаc(SO2)ЪЧМзжаЕФ2БЖ
C.ЦНКтЪБЃЌSO2ЕФзЊЛЏТЪЃКІС2>80%>ІС1
D.ИУЮТЖШЯТЃЌЦНКтГЃЪ§KЃН400
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСагаЙиЫФИіГЃгУЕчЛЏбЇзАжУЕФа№ЪіжаЃЌе§ШЗЕФЪЧ![]() ЁЁЁЁ
ЁЁЁЁ![]()
|
|
|
|
ЭМЂёМюадаПУЬЕчГи | ЭМЂђЧІаюЕчГи | ЭМЂѓЕчНтОЋСЖЭ | ЭМЂєЧтбѕШМСЯЕчГи |
A. ЭМЂёЫљЪОЕчГижаЃЌMnO2ЪЧе§МЋЃЌЕчМЋЗДгІЪНЪЧ2H2O+2e-=H2Ёќ+2OH-
B. ЭМЂђЫљЪОЕчГиЗХЕчЙ§ГЬжаЃЌЕБЭтЕчТЗЭЈЙ§1molЕчзгЪБЃЌРэТлЩЯИКМЋАхЕФжЪСПдіМг96g
C. ЭМЂѓЫљЪОзАжУЙЄзїЙ§ГЬжаЃЌбєМЋжЪСПМѕЩйСПЕШгквѕМЋЕФжЪСПдіМгСП
D. ЭМЂєЫљЪОЕчГижаЃЌВЛЙмKOHШмвКЛЛГЩH2SO4ШмвКЛЙЪЧNa2SO4ШмвКЃЌЕчГиЕФзмЗДгІЪНВЛБф
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com