
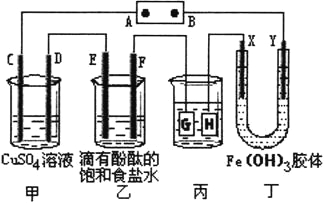
����Ŀ������ͼ��ʾ��C��D��E��F��X��Y���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ������ͨ��ǰ����Һ������䣩��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ����X����������ɫ��dz��Y����������ɫ�����ش�
��1�����ס���װ���е�C��D��E��F�缫��ֻ��һ�ֵ�������ʱ����Ӧ���ʵ����ʵ���֮��Ϊ__________��
��2�����ñ�װ�ø�ͭ����������������Һ��pH��13ʱ����ʱ����Һ���Ϊ500mL�������жƼ���������������Ϊ__________��
��3��Fe(OH)3������Ʊ����ϸ��Ҫ��С������FeCl3��Һ�еμ�NaOH��Һ���Ʊ�Fe(OH)3���壬����ܿ�������˺��ɫ�ij������������Һ��pH=5�����ʱ��Һ��c(Fe3��)=__________mol/L������֪Ksp[Fe(OH)3]=1��10��36����
��4�����ü���ȼ�ϵ�أ��������ҺΪ2L2mol/LKOH��Һ���ṩ��Դ������ͨ����飬�ڱ�״���£����ļ�������VL��������CH4�������44.8��V��89.6ʱ����ʱ��Դ��B�������ĵ缫��ӦΪ��__________��
���𰸡���1��1��2��2��2
��2��5.4g
��3��10-9
��4��CH4��8e����9CO32����3H2O=10HCO3��
��������
�������:������������Ľ��������磬��ֱ����Դ��ͨ����X����������ɫ��dz��Y����������ɫ���˵��Y�����Դ�ĸ���������YΪ�������ɵó�D��F��H��Y��Ϊ������C��E��G��X��Ϊ������A�ǵ�Դ��������B�Ǹ�����
��1��C��D��E��F�缫�����ĵ缫��Ӧ�ֱ�Ϊ��4OH-�TO2��+2H2O+4e-��Cu2++2e-�TCu��2Cl-�TCl2��+2e-��2H++2e-�TH2���������缫ת�Ƶ��Ӿ�Ϊ1molʱ�����ɵ��ʵ����ֱ�Ϊ��0.25mol��0.5mol��0.5mol��0.5mol�����Ե��ʵ����ʵ���֮��Ϊ1��2��2��2��
��2�����װ���У��Ʋ�����������������Ƽ�������������HӦ���ǶƼ�����������Һ��pH��13ʱ����ʱ����Һ���Ϊ500mL��ʱ�����ݵ缫��Ӧ2H++2e-�TH2������ŵ�������ӵ����ʵ���Ϊ��0.1mol/l��0.5L=0.05mol����ת��0.05mol����ʱ�����жƼ���������������=108g/mol��0.05mol=5.4g��
��3����֪Ksp[Fe(OH)3]=1��10��36��pH=5����Һc(H��)=10-5mol/L��c(OH��)=1��10-14��10-5=10-9mol/L����c(Fe3��)����10-9��3=1��10��36��c(Fe3��)=10-9mol/L��
��4��n��KOH��=2mol/L��2L=4mol�������Ⱥ�����Ӧ��CH4+2O2��CO2+2H2O����CO2+2KOH=K2CO3+H2O����K2CO3+CO2+H2O=2KHCO3����44.8 L��V��89.6 Lʱ��2mol��n��CH4����4mol����2mol��n��CO2����4mol��������Ӧ�٢ڢ����õ�K2CO3��KHCO3��Һ�����ܷ�ӦʽΪ��CH4 +2O2+K2CO3�T2KHCO3+H2O�������ĵ缫��ӦʽΪCH4��8e����9CO32����3H2O=10HCO3����


 ��������ѧ����ϵ�д�
��������ѧ����ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���챽��ȩ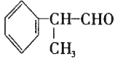 �ڹ�ҵ������Ҫ��;����ϳ��������£�
�ڹ�ҵ������Ҫ��;����ϳ��������£�

��1���챽��ȩ�����Ը�����������������л���Ľṹ��ʽ��____________��
��2���ںϳ����������ķ�Ӧ������____________����Ӧ��������������____________��
��3����Ӧ���Ļ�ѧ����ʽΪ____________��
��4���챽��ȩ����������Ӧ�Ļ�ѧ����ʽΪ__________��
��5��D�������л���X��һ�������¿�����һ����Է�������Ϊ178���������ʣ���X��������____________��D�����ж���ͬ���칹�壬�������㱽����������ȡ����������ʹFeCl3��Һ����ɫ��ͬ���칹����____________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ����������Ԫ�����ڱ��е�λ�����£��ش��й����⣺
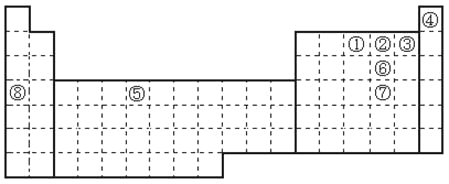
��1��д����������Ԫ���е�һ����������Ԫ�ط���___________��
��2��д���ڢޢ�����Ԫ����̬�⻯ѧ�ķе�˳��___________���������ʽ˳��
��3���뻭��[Cu��NH3��4]2+���ӽṹʾ��ͼ___________��
��4���뻭��������Χ�����Ų�ͼ___________��
��5��SeO3�Ŀռ乹��Ϊ___________��
��6��SeO32�����ӵ�VSEPR����Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧƽ��ԭ������ѧ��ѧѧϰ����Ҫ���ݣ���ش��������⣺
�״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ�Ͽ��úϳ�������Ҫ�ɷ�CO��H2���Ʊ��״���
��1����֪��CO��H2��CH3OH����ȼ��������H���ֱ�Ϊ-283.0kJ/mol��-241.8kJ/mol��-192.2 kJ/mol����д���ϳ����Ʊ��״����Ȼ�ѧ����ʽ ��
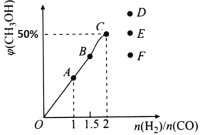
��2�����ھ��ȡ����ݵ��ܱ������г���1 mol CO��2 mol H2������CO��g��+2H2��g��![]() CH3OH��g����Ӧ������ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ��Ϊƽ��״̬����______����ѡ����ĸ����
CH3OH��g����Ӧ������ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ��Ϊƽ��״̬����______����ѡ����ĸ����
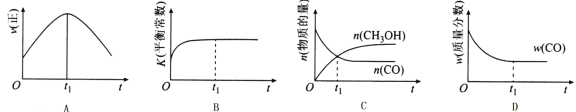
��3����T1��ʱ�������Ϊ5 L�ĺ��������г���3 mol�ĺϳ�������Ӧ�ﵽƽ��ʱCH3OH�����������n��H2����n��CO���Ĺ�ϵ��ͼ��ʾ��H2��CO��2:1Ͷ��ʱ����5 min�ﵽƽ�⣬��5 min����H2��ʾ�ķ�Ӧ����Ϊv��H2��=_______���¶Ȳ��䣬��![]() ʱ���ﵽƽ��״̬��CH3OH���������������ͼ���е�______�㡣
ʱ���ﵽƽ��״̬��CH3OH���������������ͼ���е�______�㡣
��4�����м״��ķ�ˮ�����ŷŻ����ˮ��Ⱦ������ClO2��������ΪCO2��Ȼ���ټӼ��кͼ��ɡ�д�������״����Է�ˮ�����У�ClO2��״���Ӧ�����ӷ���ʽ��________________________��
��5��ˮ����ż����ɱ�ʾΪH2O+H2O![]() H3O++OH-����ˮ�������ƣ��״�Ҳ�ܷ�����ż���룬��д���״�����ż���뷽��ʽ_______________________________________�����״��м������������Ʒ�Ӧ���ɼ״��ƣ���Ӧ��Ļ��Һ�еĵ���غ�ʽ_____________________________��
H3O++OH-����ˮ�������ƣ��״�Ҳ�ܷ�����ż���룬��д���״�����ż���뷽��ʽ_______________________________________�����״��м������������Ʒ�Ӧ���ɼ״��ƣ���Ӧ��Ļ��Һ�еĵ���غ�ʽ_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ���(S2Cl2)�ǹ㷺������ҵ����������ӽṹ����ͼ��ʾ�������£�S2Cl2��һ�ֳȻ�ɫ��Һ�壬��ˮ��ˮ�⣬��������ʹƷ����ɫ�����塣����˵���д������( )

A��S2Cl2�ĵ���ʽΪ![]()
B��S2Cl2Ϊ���м��Լ��ͷǼ��Լ��ķǼ��Է���
C��S2Br2��S2Cl2�ṹ���ƣ��۷е㣺S2Br2>S2Cl2
D��S2Cl2��H2O��Ӧ�Ļ�ѧ����ʽ����Ϊ��2S2Cl2 ��2H2O===SO2����3S����4HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��50ml0��50mol��L-1������50mL0��55mol��L-1NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȡ��ش��������⣺
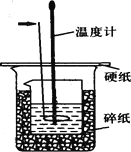
��1����ͷ��ָ������������ �������� ��
��2��ʵ�����õ�NaOH��Һ�����������ͬ����Ũ��ȴ��������ԭ���� ��
��3������ͬŨ�Ⱥ�����Ĵ������HCl��Һ��������ʵ�飬�ų��������� ��������ƫ������ƫС��������Ӱ����������õ���H�� ������ƫ������ƫС��������Ӱ��������
��4����ͼ��ʾʵ��װ�ô�����һ������������� ��
��5��ʵ�����к�����H =" -" 57.3 kJ��mol�C1��������������ʧ����������ʵ��ǰ���¶ȵIJ�ֵ��t ������һλС����ˮ�ı�����c=4.18J/��g������������Һ���ܶȽ���ȡ1g/ml ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ԫ����(HnX)��Һ��c(H��)���Ƚ���������ǿ��ʱ��ͨ��ֻ���ǵ�һ�����롣�ش����й��ڶ�Ԫ����HnX�����⡣
��1����ҪʹHnX��Һ��c(H��)/c(HnX)�����Բ�ȡ�Ĵ�ʩ��__________��
A�������¶� B����������̬HnX C��������NaOH��Һ D����ˮ
��2�������ӷ���ʽ����NanX�ʼ��Ե�ԭ��______________________________��
��3����HnXΪH2C2O4����ij�¶��£�H2C2O4��K1=5��10��2��K2=5��10��5������¶��£�0.2mol/L H2C2O4��Һ��c��H����ԼΪ__________mol/L������ȷ���㣬�Ҽ�֪![]() ��
��
��4����֪KHC2O4��Һ�����ԡ�
��KHC2O4��Һ�У�������Ũ���ɴ�С��˳����____________________��
����KHC2O4��Һ�У�������Ũ�ȹ�ϵ��ȷ����__________��
A��c(C2O42��)��c(H2C2O4)
B��c(OH��)=c(H��)��c(HC2O4��)��2c(H2C2O4)
C��c(K��)��c(H��)=c(OH������c(HC2O42��)��2c(C2O42��)
D��c(K��)=c(C2O42��)��c(HC2O4��)��c(H2C2O4)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ���������������X��Y��Z��G��Q��R��T����Ԫ�أ��˵������С��36����֪X��Y��Z��Ԫ�صĻ�̬ԭ��2p�ܼ����е����ӣ������Ӹ����ֱ���2��3��2��G��Tԭ���������18��TԪ�������ڱ���ds���ĵ�һ��Ԫ�أ�Qԭ��s�ܼ���p�ܼ���������ȣ�R������������ּ���������Ӳ�Ʒ�ĺ��IJ��ϡ�
��1��Yԭ�Ӻ����________�ֲ�ͬ�˶�״̬�ĵ��ӣ�TԪ�ػ�̬ԭ����________�ֲ�ͬ�ܼ��ĵ��ӡ�________����״��ͬ��ԭ�ӹ����
��2��X��Y��Z�ĵ縺���ɴ�С��˳��Ϊ________��G��Q��R��һ��������С�����˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
��3��YԪ�ػ�̬ԭ�ӵĵ����Ų�ͼ________��TԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽ________��
��4��XZ2�ĵ���ʽΪ________���õ��뷽��ʽ��ʾY������⻯���ˮ��Һ�ʼ��Ե�ԭ��________��
��5��+1����̬��������ʧȥһ�������γ�+2����̬��̬����������Ҫ��������Ϊ�ڶ�������I2�����λ���I3��I4��I5���������Ʋ�GԪ�صĵ�����ͻ��Ӧ�����ڵ�________�����ܡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����HI(g)�����ܱ������У�ij�¶��·������б仯��2HI(g) ![]() H2(g)+I2(g)��H��0
H2(g)+I2(g)��H��0
��1���÷�Ӧƽ�ⳣ���ı���ʽΪK=______________����H2(g)+I2(g) ![]() 2HI(g)ƽ�ⳣ���ı���ʽΪK1=_____________(��K��ʾ)��
2HI(g)ƽ�ⳣ���ı���ʽΪK1=_____________(��K��ʾ)��
��2������Ӧ�ﵽƽ��ʱc(I2)=0.5mol/L��c(HI)=4mol/L����c(H2)Ϊ________��HI�ķֽ���Ϊ________��
��3�����жϸ÷�Ӧ�ﵽƽ��״̬��������________
A��������ѹǿ����
B�����������c(HI)����
C��c(I2)=c(H2)
D��v(HI)��=v(H2)��
��4�����÷�Ӧ800��ʱ�ﵽƽ��״̬����ƽ�ⳣ��Ϊ1.0��ijʱ�̣���������ڸ����ʵ�Ũ���ֱ�Ϊc(HI)=2.0mol/L��c(I2)=1.0mol/L��c(H2)=1.0mol/L�����ʱ�̣���Ӧ��_________(������������������������ͬ)���У��������¶ȣ���Ӧ��_________���С�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com