
����Ŀ��ʵ����������ͼװ�ý����к��ȵIJⶨ���ش��������⣺
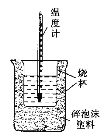
(1)��ͼ��������δ������������____________��____________��
(2)�ڲ�����ȷ��ǰ��������к��Ȳⶨ��ȷ�ԵĹؼ���____________��
(3)�����0.50 mol/L��������������ƹ������ʵ�飬��ʵ��������������к�������____________(����ƫ��������ƫС������������)��ԭ����____________��
(4)�Ҵ���δ����ȼ������ѡ������Һ��ȼ�ϡ�2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43 kJ����������ʾ�Ҵ�ȼ���ȵ��Ȼ�ѧ����ʽΪ____________________________��
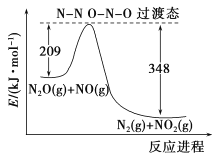
(5)��N2O��NO��Ӧ����N2��NO2�������仯��ͼ��ʾ��������1molN2������H=__________kJ��mol-1��
���𰸡����β�������� ֽ��� ���� ƫ�� �������ƹ����ܽ���� ![]() -139
-139
��������
(1)�������ȼƵĹ������жϸ�װ�õ�ȱ��������
(2)�������кͷ�Ӧ�У�����ȷ��������ɢʧ��
(3)�������ƹ�������ˮ�ų�������
(4)ȼ������ָ1mol��ȼ����ȫȼ�������ȶ���������ų����������Ҵ���ȫȼ�����ɶ�����̼��Һ̬ˮ������2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43kJ������������1mol�Ҵ�ȼ����ȫȼ������Һ̬ˮ�ų����������ݴ���д�Ҵ�ȼ���ȵ��Ȼ�ѧ����ʽ��
(5)��ͼ��֪������Ӧ�Ļ��Ϊ209kJ��mol-1���淴Ӧ�Ļ��Ϊ348kJ��mol-1������ͼ��֪���˷�ӦΪ���ȷ�Ӧ�������淴Ӧ�Ļ��ֻ����Ƿ�Ӧ���ʱ䡣
(1)���к��Ȳⶨʵ��Ĺ����֪����װ�õ�ȱ�������ǻ��β����������ֽ��ǣ�
(2)���кͷ�Ӧ�У�����к��Ȳⶨ��ȷ�ԵĹؼ���ȷ��������ɢʧ�������£�
(3)�������ƹ�������ˮ���ȣ��¶�ƫ�ߣ�����ʵ���в�õ��к�����ֵ��ƫ��
(4)�Ҵ���ȫȼ�����ɶ�����̼��Һ̬ˮ������2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43kJ����������1mol�Ҵ���ȫȼ������Һ̬ˮ�ų�������Ϊ��1366.89kJ������ȼ���ȵ��Ȼ�ѧ����ʽΪ��C2H5OH(l)+3O2(g)�T2CO2(g)+3H2O(l)��H=1366.89kJmol1��
(5)��ͼ��֪���÷�ӦΪ���ȷ�Ӧ���˷�Ӧ�ķ�Ӧ��Ϊ���淴Ӧ�Ļ��֮����H=(209-348)kJ��mol-1=-139kJ��mol-1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F������ԭ�������������������Ԫ�أ�AԪ�ص�ԭ�������ڱ��а뾶��С�ģ�BԪ�ص��������������ڲ��2����DԪ�ص��������������������������3����E��Aͬ���壻F�����������γ��������Ҫ�ɷ�֮һ����ش��������⣺
(1)FԪ�ص�����________
(2)A��D��E�γɵĻ������������______ (�������ӻ������������ۻ�������)
(3)�õ���ʽ��ʾBD2���γɹ���____________
(4)B��C������������ˮ���������________��________(�ѧʽ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NaOH��Na2CO3�����Һ�еμ�0.1mol��L��1ϡ���ᣬCO2���������������������(V)�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ����
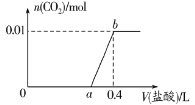
A.��0��a��Χ�ڣ�ֻ�����кͷ�Ӧ
B.a��0.2
C.ab�η�����Ӧ�����ӷ���ʽΪCO32����2H��=CO2����H2O
D.ԭ�����Һ��NaOH��Na2CO3�����ʵ���֮��Ϊ2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͭ�ķ�ĩ�����ʵ���֮��Ϊ2�U1�ı�����Ͼ��ȣ�ȡ��mg����Ͷ�뵽100mL 3mol��L-1FeCl3��Һ�У���ַ�Ӧ����Ӧ����Һ����仯���Բ��ƣ����Լ��㣺
��1�����������ȫ�ܽ⣬��m���ֵΪ____��
��2�������Һ��Cu2+Ũ��Ϊ0.25 mol��L-1����mֵΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ����±���
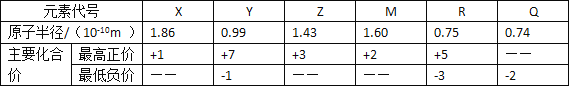
����˵����ȷ����( )
A.���Ӱ뾶��r(R3��)��r(X��)��r(Z3��)
B.Ԫ��X ��Q �γɵĻ������в����ܺ��й��ۼ�
C.Q2���� R3��������ʧȥ����
D.Y �ĺ���������һ���� R �ĺ����������ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽����ɫ����X(��������Ԫ��)����ɺ����ʣ���Ʋ��������ʵ�飨�����Լ����ǹ����ģ���
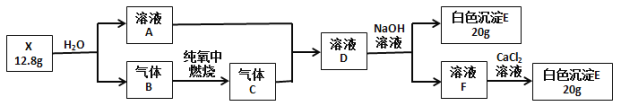
��������B�ڱ�״���µ��ܶ�Ϊ1.16 g��L-1��ش�
(1) X�Ļ�ѧʽ��________��
(2) ����B�ĵ���ʽ________��
(3) ��ҺD��NaOH��Һ��Ӧ�����ӷ�Ӧ����ʽ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���йؾ���Ľṹ��ͼ��ʾ������˵���в���ȷ���ǣ� ��
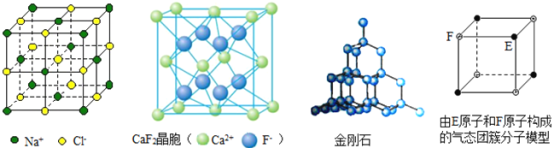
A. ��NaCl��������Na+�����Cl-��6��
B. ��CaF2�����У�ÿ������ƽ��ռ��4��Ca2+
C. �ڽ��ʯ�����У�̼ԭ����̼̼�������ı�Ϊ1��2
D. ����̬�Ŵط��ӵķ���ʽΪEF��FE
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ�����������ʵ�鷽�����ó����Լ��Ľ��ۣ�����ʵ�鷽����Ƽ����۾���ȷ����(����)
A. ������Һ![]() ˮ��Һ��Һ
ˮ��Һ��Һ![]() ���������ۣ�������ȫû��ˮ��
���������ۣ�������ȫû��ˮ��
B. ������Һ![]() ˮ��Һ
ˮ��Һ![]() ��ש��ɫ���������ۣ�������ȫˮ��
��ש��ɫ���������ۣ�������ȫˮ��
C. ������Һ![]() ˮ��Һ
ˮ��Һ![]() �к�Һ
�к�Һ![]() ��ש��ɫ���������ۣ�������ˮ��
��ש��ɫ���������ۣ�������ˮ��
D. ������Һ![]() ˮ��Һ
ˮ��Һ![]() �������ۣ�����û��ˮ��
�������ۣ�����û��ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϳɰ��ķ�Ӧ���£�3H2+N2![]() 2NH3��ij�¶��£����ݻ��㶨Ϊ2.0 L���ܱ������г���2.0 mol N2��2.0 mol H2��һ��ʱ���Ӧ��ƽ��״̬��ʵ���������±���ʾ��
2NH3��ij�¶��£����ݻ��㶨Ϊ2.0 L���ܱ������г���2.0 mol N2��2.0 mol H2��һ��ʱ���Ӧ��ƽ��״̬��ʵ���������±���ʾ��
t/s | 0 | 50 | 150 | 250 | 350 |
n(NH3)/mol | 0 | 0.24 | 0.36 | 0.40 | 0.40 |
(1)0��50 s�ڵ�ƽ����Ӧ���� v(N2)��_______________��
(2)250 sʱ��H2��ת����Ϊ_______________��
(3)��֪N��N�ļ���Ϊ946 kJ��mol-1��H��H�ļ���Ϊ436 kJ��mol-1��N��H�ļ���Ϊ391 kJ��mol-1��������1 mol NH3�����е������仯Ϊ_______kJ����ͼ����ȷ��ʾ�÷�Ӧ�������仯����_____������ĸ����
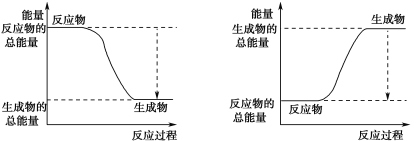
A B
(4)Ϊ�ӿ췴Ӧ���ʣ����Բ�ȡ�Ĵ�ʩ______________��
a�������¶� b������ѹǿ c������ʱ����He��
d����ѹʱ����He�� e����ʱ�����NH3
(5)����˵���������____________��
a��ʹ�ô�����Ϊ�˼ӿ췴Ӧ���ʣ��������Ч��
b�����������£�N2������100%ת��ΪNH3
c����һ�������£��ϳɰ���Ӧ��һ������
d��250��350 sʱ��������Ũ�ȱ��ֲ��䣬��Ӧֹͣ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com